The broad picture of interstitial cystitis
Interstitial cystitis (IC) is a controversial diagnosis that has become muddied and oversimplified. It was originally described as a distinct ulcer (Hunner’s lesion) seen in the bladder on cystoscopy, the treatment of which often led to symptomatic relief. Hunner’s lesion IC is the “classic” form of IC and should be considered a separate disease; it is not a progression of nonulcerative interstitial cystitis/painful bladder syndrome (IC/BPS).
Only a fraction of patients with the key symptoms of IC/BPS – urinary frequency, urgency, and pelvic pain – have ulcers within the bladder. And many of the patients who are diagnosed with IC/BPS are found not to have bladder pathology as the name implies, but rather pelvic floor dysfunction. That the bladder is often an innocent bystander to a larger process means that, as clinicians, we must be thoughtful and astute about our diagnostic process.
Hunner’s lesions
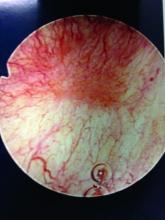
Patients with Hunner’s lesions have a rapid onset of symptoms, typically are older, and have a visible lesion in their bladder that almost always is on the dome or lateral walls. The lesion is often erythematous with central vascularity and mucosal sloughing.
The bladder is a storage organ and urine is toxic. The exposed ulcer results in severe pain with bladder filling and also pain at the end of voiding as the bladder collapses, causing ulcerated tissue to come into contact with other sections of the bladder wall and sending a “jolt” of pain through the pelvis.
If the initial cystoscopy demonstrates inflammatory-appearing lesions or ulcerations suggestive of Hunner’s lesions, I will still do a hydrodistension. By stretching the bladder, the lesions typically expand, crack, and bleed. This helps define the entire diseased area and shows what areas of the bladder need to be cauterized to seal the ulcers and destroy the exposed nerve endings. If this is a new diagnosis, the lesion should be biopsied after the hydrodistension to rule out carcinoma.
Hunner’s lesions can lead to rapid disease progression due to chronic inflammation and subsequent collagen deposition and scarring. Even on initial diagnosis of Hunner’s lesions, a capacity of 350 cc or less (compared with 1,100 cc in a normal bladder) on hydrodistension under anesthesia is not uncommon. This markedly reduced bladder capacity may lead to end-stage bladder impacting the kidneys and requiring a urinary diversion.
Eradicating the ulcers with resection or cautery often results in marked and immediate improvement in bladder pain, albeit not long-lasting. I will typically place a resectoscope and use a roller ball at 25 watts of current. The entire ulcerated areas are cauterized by rapidly rolling the ball over the area of inflammation and avoiding a deep thermal burn. The goal is to seal the ulcer and destroy the exposed nerve endings so that urine can no longer act as an irritant. Recurrence in 6 months to 1 year is common and retreatment is almost always necessary. We have demonstrated, however, that recurrent cautery of ulcers does not lead to smaller anesthetic bladder capacities (Urology. 2015 Jan;85[1]:74-8).
Low-dose cyclosporine can be very effective at reducing Hunner’s lesion recurrence and improving storage symptoms (Exp Ther Med. 2016 Jul;12[1]:445-50). I use 100 mg twice a day for a month and then 1 pill a day thereafter. This is a relatively low dose, but hypertension can be a side effect and blood pressure should be monitored along with routine labs.
The broader picture
Hunner’s lesion IC is pretty straightforward and clearly a bladder disease. However, in recent years the term IC/BPS has been broadly used to describe women who have symptoms of pelvic pain, urinary urgency, and frequency, but no true bladder pathology to explain their symptoms. One problem: There is no definitive diagnostic test or evidence-based diagnostic process for IC/BPS. In fact, the diagnosis section of the American Urological Association guideline on diagnosis and treatment of IC/BPS, last updated in 2015, is almost entirely consensus-based (J Urol. 2015 May;193[5]:1545-53). It largely remains a diagnosis of exclusion.
As the AUA guidelines state, a careful history, physical examination, and laboratory assessment are all important for documenting symptoms and signs and ruling out significant causes of the symptoms. I frequently see patients who have been diagnosed with IC who have frequency and urgency but no pain (in which case overactive bladder should be considered) or who have pelvic pain but no bladder symptoms, again likely not IC. Pain that worsens with bladder filling and improves after bladder emptying is typical of IC/BPS. This finding in the absence of other confusing symptoms supports the diagnosis of IC/BPS.
It has become too easy for the average clinician to apply a label of IC/BPS to a patient complaining of pelvic pain; this often results in the patient undergoing invasive and nonhelpful therapies such as cystoscopy, hydrodistension, urodynamics, bladder instillations, and other bladder-directed therapies.
More than 20 years of research supported by the National Institutes of Health and industry have failed to show that bladder-directed therapy is superior to placebo. This fact suggests that the bladder may be an innocent bystander in a larger pelvic process. As clinicians, we must be willing to look beyond the bladder and examine for pelvic floor issues and other causes of patient’s symptoms and not be too quick to begin bladder-focused treatments.
A number of disease processes – such as recurrent urinary tract infection, urethral diverticulum, endometriosis, and pudendal neuropathy – can mimic the symptoms of IC/BPS. The most common missed diagnosis in the IC patient is pelvic floor dysfunction that results in a hypertonic contracted state of the levator muscles – a chronic spasm, in essence – that in turn leads to decreased muscle function, increased myofascial pain, and myofascial trigger points (Curr Urol Rep. 2006 Nov;7[6]:450-5).
We and others have reported that up to 85% of patients labeled with IC/BPS have been found on examination to have pelvic floor dysfunction or a diffuse pelvic floor hypersensitivity. The pelvic floor is important in maintaining healthy bladder, bowel, and sexual function. If the pelvic floor is in spasm, this can result in urinary frequency, hesitancy, and pelvic pain.
Many of these patients with contracted pelvic floor muscles report pain with sexual intercourse – often so severe as to cause abstention. In fact, when patients answer no to the question of whether they have pain with intercourse, I know it is unlikely that they have significant pelvic floor dysfunction. This is a key question for history taking.
Other key questions concern the impact of stress on symptoms and a history of any type of abuse. In a study we conducted about 10 years ago, we found that among 76 women who were diagnosed with IC and subsequently evaluated in our clinic, almost half (49%) reported abuse (emotional, physical, and/or sexual). The vast majority (85%) had levator pain (J Urol. 2007 Sep;178[3 Pt 1]:891-5).
Other types of stress – from past surgeries to traumatic life events – may similarly serve as triggers or precursors to pelvic floor dysfunction in some women. I often tell patients that people put stress in different areas of their bodies. While some get tension headaches or low-back aches, others get pelvic pain from contracting and guarding the levator muscles.