Intramedullary spinal cord and leptomeningeal metastases presenting as cauda equina syndrome in a patient with melanoma
Accepted for publication August 1, 2016
Correspondence Stephanie Lindauer, MD; Lindauers3@uthscsa.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(4):e224-e227
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0293
Submit a paper here
The incidence of malignant melanoma has been rising in the United States, especially among non-Hispanic white men and women. Death rates have increased for those aged 65 years or older, and incidence rates have increased for all age groups.1 It is a serious public health issue.
Given the unique biology of melanoma, metastatic disease can present in a variety of ways. In most cases, the lymph nodes and lungs are involved.2 The incidence of brain metastases is 10%-40%, however the percentage may be even higher based on reported incidence of autopsy reports.3 The most common forms of metastatic melanoma to the spine are vertebral and intramedullary.4 Specifically, leptomeningeal involvement can be found in 20% of patients in clinical studies and 44%-70% in autopsy series of patients with central nervous system (CNS) metastatic disease.5 Despite its incidence, leptomeningeal disease (LMD) from melanoma is rarely discussed in the literature and the diagnosis may be difficult. Even rarer is the documented presentation of intramedullary spinal cord metastases, or “drop metastases.”6 In our review of the literature, we found no published case reports to date of drop metastases from melanoma causing cauda equina syndrome.
The prognosis of patients with metastatic melanoma with brain metastases is very poor, with a median overall survival of about 4 months reported in several studies.7-9 Prognosis is even worse for patients with leptomeningeal involvement, and median survival without therapy is about 4-6 weeks.10 A combination of intrathecal and systemic chemotherapy can be used to treat LMD.11
Case presentation and summary
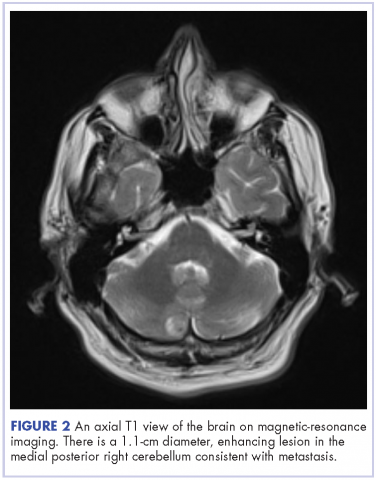
This is the case of a 56-year-old man with history of metastatic melanoma that had been initially diagnosed about 4 years before the current case presentation. Original sites of disease were a supraclavicular lymph node and solitary liver metastasis, both of which were resected. The patient then developed biopsy-proven lung involvement that required left and right wedge resections. Mutation testing for BRAF V600E and BRAF V600K was sent and not detected. Therefore the patient did not receive any BRAF-targeted therapies. Subsequently, recurrent metastatic disease to the brain with 2 dominant lesions in the cerebellum and the occiput as well as numerous small lesions at the gray-white matter junction was identified (Figure 1 and Figure 2).
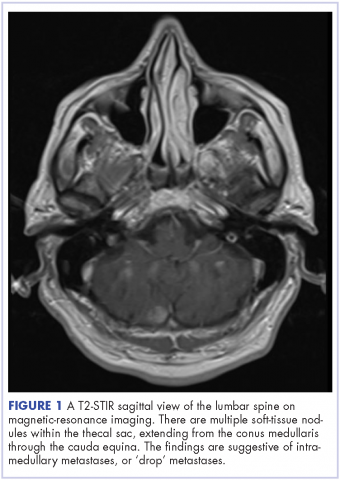
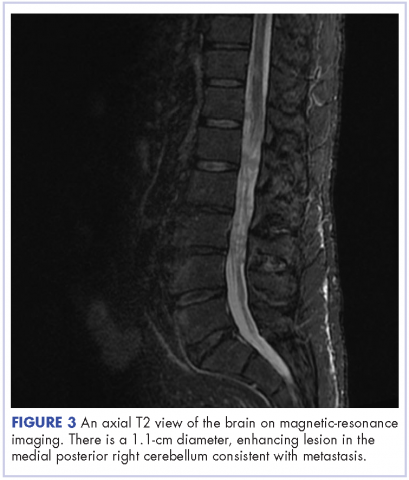
The patient received whole-brain radiation (30 Gy in 10 fractions of 3 Gy each). There was no evidence of disease in his spine at that time. About 2 weeks after completing whole-brain radiation, the patient presented to the hospital with left lower extremity weakness, urinary retention, bowel incontinence, saddle anesthesia, and malaise. The symptoms had begun after he had finished whole-brain radiation and weakness progressed to the point at which he need a cane to be able to walk. A physical examination was significant for hyporreflexia, decreased strength and sensitivity on left lower extremity, saddle anesthesia, and lumbar spinal tenderness to palpation. The results of magnetic-resonance imaging (MRI) of the spine revealed multiple soft-tissue nodules extending from the conus medullaris throughout the cauda equina, consistent with intramedullary metastases, as well as concomitant leptomeningeal involvement (Figure 3).
The patient was started on steroids with minimal improvement in neurologic function. We consulted with our neurosurgery colleagues, but learned that no direct surgical intervention could be performed because of widespread involvement. We then proceeded with radiation, 30 Gy in 10 fractions to the lumbar spine. Intrathecal chemotherapy with methotrexate (12 mg twice a week) was also started, with a plan to complete 4 weeks. Shortly after starting radiation therapy and methotrexate, we observed clinical improvement in the patient, with mildly increased left lower extremity strength and increased ambulation with a physical therapist.
Cerebrospinal fluid studies (CSF) showed clearance of malignant cells after 2 treatments of intrathecal methotrexate as well as improvement in CSF chemistry parameters: the patient’s protein level decreased from 1,095 mg/dL to 42 mg/dL (15-45 mg/dL) and his glucose level increased from 3 mg/dL to 73 mg/dL (40-85 mg/dL) However, after completing 3 weeks of intrathecal chemotherapy, the hospital course was complicated by leukopenia, thrombocytopenia, and spontaneous intracranial hemorrhage. The cytopenias were thought to be secondary to systemic effect of intrathecal methotrexate in conjunction with the radiation treatments to the spine. Intrathecal chemotherapy was held.
The patient was not a candidate for systemic immunotherapy because of his decline in performance status. He continued to deteriorate neurologically, and the family decided to pursue inpatient hospice. He died a week after transfer to hospice and 5 weeks after the initial diagnosis of leptomeningeal and intramedullary metastases.