Clinical Assessment and Management of Cancer-Related Fatigue
From the University of Texas MD Anderson Cancer Center, Houston, TX.
Abstract
- Objective: To review the evidence on interventions for managing cancer-related fatigue (CRF) and provide evidence-based guidance on approaches to its management.
- Methods: Nonsystematic review of the literature.
- Results: Several theories have been proposed to explain the biology of CRF, but there is no single clear mechanism that can be targeted for therapy. The approach to patients begins with screening for fatigue and assessing its intensity, followed by a thorough history and examination to determine whether any reversible medical conditions are contributing to fatigue. Management of underlying medical comorbidities may help some patients. For patients whose fatigue persists, pharmacologic and nonpharmacologic treatment options are available. Pharmacologic options include psychostimulants, such as methylphenidate and modafinil, and corticosteroids. Nonpharmacologic approaches include exercise, cognitive behavior therapy, yoga, acupuncture, and tai chi.
- Conclusion: We recommend an individualized approach, often with a combination of the available options. Patients need to be evaluated periodically to assess their fatigue, and since cancer-related fatigue affects survivors, long-term follow-up is needed.
Key words: fatigue; cancer; pro-inflammatory cytokines; nonpharmacologic; psychostimulants.
Fatigue is a common distressing effect of cancer [1].It impairs the quality of life of patients undergoing active cancer treatment and of post-treatment survivors. The National Comprehensive Cancer Network (NCCN) defines cancer-related fatigue (CRF) as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning [2].” Differences between CRF and fatigue reported by individuals without cancer are that CRF is more severe and is not relieved by rest. The prevalence of CRF in cancer patients and survivors is highly variable, ranging between 25% and 99% [2,3]. This variability may be secondary to methods used for screening fatigue and characteristics of the patient groups. In this article, we discuss recognition of CRF and approaches to its management.
Pathophysiology
The specific pathophysiologic mechanism underlying CRF is unknown, making targeted treatment a challenge. The multidimensional and subjective nature of CRF has limited the development of research methodologies to explain this condition. However, research has been done in both human and animal models, and several theories have been proposed to explain the pathophysiology of CRF. While pro-inflammatory cytokines remain the central factor playing a significant role at multiple levels in CRF, there may be a complex interplay of more than 1 mechanism contributing to fatigue in an individual patient.
Central Nervous System Disturbances
The basal ganglia are known to influence motivation. Lack of motivation and drive may cause failure to complete physical and mental tasks, even with preserved cognitive ability and motor function. In a study of melanoma patients receiving interferon, increased activity of the basal ganglia and the cerebellum resulted in higher fatigue scores [4]. Higher levels of cytokines may alter blood flow to the cerebellum and lead to the perception of fatigue. In a study of 12 patients and matched controls, when patients were asked to perform sustained elbow flexion until they perceived exhaustion, CRF patients perceived physical exhaustion sooner than controls. In CRF patients in this study, muscle fatigue measured by electromyogram was less than that in healthy individuals at the time of exhaustion, suggesting the role of the central nervous system in CRF [5]. However, there is not enough evidence at this time to support central nervous system disturbance as the main contributing factor to fatigue in cancer patients.
Circadian Rhythm Dysregulation
Circadian rhythm is regulated by the suprachiasmatic nucleus in the hypothalamus through cortisol and melatonin. Sleep disturbances occur with disruption of the circadian rhythm. Tumor-related peptides such as epidermal growth factor or alterations in serotonin and cortisol can influence the suprachiasmatic nucleus and the complex signaling pathways [2]. Positive feedback loops that are activated by cortisol under the influence of cytokines may lead to continuous cytokine production and altered circadian rhythm. Bower et al showed that changes in the cortisol curve influence fatigue in breast cancer survivors [6]. These patients had a late evening peak in cortisol levels, compared with an early morning peak in individuals without cancer.
Inhibition of Hypothalamic–Pituitary–Adrenal Axis
The hypothalamic–pituitary–adrenal (HPA) axis regulates the release of the stress hormone cortisol. One of several hypotheses advanced to explain the effect of serotonin and the HPA axis on CRF suggests that lower serotonin levels cause decreased activation of 5-hydroxytrytophan 1-a (5-HT1-a) receptors in the hypothalamus, leading to decreased activity of the HPA axis [6]. The inhibition of the HPA axis may occur with higher levels of serotonin as well [7]. The 5-HT1-a receptors are also triggered by cytokines. However, the correction of serotonin levels by antidepressants was not shown to improve fatigue [8]. Inhibition of the HPA axis can also lead to lower testosterone, progesterone, or estrogen levels, which may indirectly contribute to fatigue [2].
Skeletal Muscle Effect
Chemotherapy- and tumor-related cachexia have a direct effect on the metabolism of skeletal muscles. This effect may lead to impaired adenosine triphosphate (ATP) generation during muscle contraction [9]. ATP infusion improved muscle strength in one trial, but this was not confirmed in another trial [10,11]. Muscle contraction studies showed no differences in the contractile properties of muscles in fatigued patients who failed earlier in motor tasks and healthy controls [12]. This finding suggests that there could be a failure of skeletal muscle activation by the central nervous system or inhibition of skeletal muscle activity. Cytokines and other neurotransmitters activate vagal efferent nerve fibers, which may lead to reflex inhibition in skeletal muscles [13,14].
Pro-inflammatory Cytokines
Tumors or treatment of them may cause tissue injury, which triggers immune cells to release cytokines, signaling the brain to manifest the symptom fatigue. Inflammatory pathways are influenced by psychological, behavioral, and biological factors, which play a role as risk factors in CRF. Interleukin 6 (IL-6), interleukin-1 receptor antagonist, interleukin-1, and tumor necrosis factor (TNF) have been shown to be elevated in fatigued patients being treated for leukemia and non-Hodgkin lymphoma [15]. IL-6 was also associated with increased fatigue in breast cancer survivors [16]. Similar findings were reported in patients undergoing stem cell transplantation and high-dose chemotherapy [17]. Elevated levels of IL-6 and C-reactive protein were also linked to fatigue in terminally ill cancer patients [18,19]. Furthermore, TNF-α signaling was associated with post-chemotherapy fatigue in breast cancer patients [20]. Leukocytes in breast cancer survivors with fatigue also have increased gene expression of pro-inflammatory cytokines, emphasizing the role of cytokines and inflammation in the pathogenesis of CRF [21].
Other Hypotheses
Several other hypotheses for CRF pathogenesis have been proposed. Activation of latent viruses such as Epstein-Barr virus, lack of social support [22], genetic alterations in immune pathway [23], epigenetic changes [24], accumulation of neurotoxic metabolites and depletion of serotonin by indoleamine 2,3-dioxygenase pathway activation [25], elevated vascular endothelial growth factor levels [26], and hypoxia-related organ dysfunction due to anemia or hemoglobin dysfunction [13] all have been postulated to cause CRF.
Approach to Evaluation and Treatment
Screening
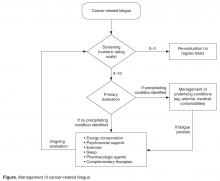
Because patients and health care professionals may be unaware of the treatment options available for CRF, patients may not report fatigue levels to their clinicians, and clinicians may not understand the impact of fatigue on their patients’ quality of life. This leads to underrecognition of the problem. The NCCN recommends screening every cancer patient and post-treatment survivor for fatigue [2]. Patients should be screened at their first visit and then at periodic intervals during and after cancer treatment.
Many scales are available to screen patients for CRF in clinical practice and clinical trials [27]. A single item that asks patients to rate their fatigue on a scale from 0 to 10—in which 0 indicates no fatigue, 1 to 3 indicates mild fatigue, 4 to 6 indicates moderate fatigue, 7 to 9 indicates severe fatigue, and 10 indicates the worst fatigue imaginable—is commonly used to screen for CRF [2]. This scale was adapted from the MD Anderson Symptom Inventory scale and is based on a large nationwide study of cancer patients and survivors [28]. The statistically derived cutoff points in this study are consistent with other scales such as the Brief Fatigue Inventory (BFI) and support the cutoff points (4–6 for moderate and ≥ 7 for severe fatigue) used in various fatigue management guidelines. Furthermore, studies of fatigue in cancer patients have revealed a marked decrease in physical function at levels of 7 or higher, suggesting 7 as an optimal cutoff to identify severe fatigue [29,30]. The Visual Analog Scale is another simple-to-use tool that helps in understanding variations in fatigue throughout the course of the day [31]. The 9-item BFI is often used in clinical trials [29]. It measures the severity of fatigue over the previous 24 hours and has been validated in non-English speaking patients [32].