'Transcutaneous Systemic' Clobetasol Reverses Bullous Pemphigoid
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
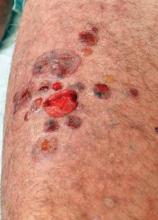
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
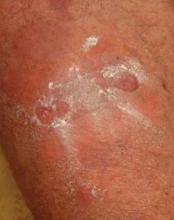
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.