Scratching Beneath the Surface
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similar to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
© 2018 Society of Hospital Medicine
A 62-year-old man with severe chronic obstructive pulmonary disease (COPD; forced expiratory volume during the first second [FEV1] 40% predicted) and type 2 diabetes mellitus presented to a Veterans Affairs emergency department (ED) with a steadily worsening cough of 4-months’ duration. He also reported subjective fevers, sputum production, shortness of breath, and unintentional 20-pound weight loss. He denied chills, chest pain, nausea, or vomiting.
Cough is classified as acute, subacute, or chronic based on duration of less than 3 weeks, between 3-8 weeks, and greater than 8 weeks, respectively. Common causes of chronic cough include bronchitis, acid reflux, cough-variant asthma, and a side effect of angiotensin converting enzyme inhibitors. Unintentional weight loss suggests a serious disorder, including indolent infection, end-stage COPD, malignancy, and autoimmune causes. Among patients with chronic bronchitis, the microbiology of sputum is often mixed with commensal respiratory flora, including Streptococcus pneumoniae and Haemophilus species. When these organisms are not recovered in sputa, or when patients fail to respond to empiric treatment, the differential diagnosis should be broadened to include pulmonary tuberculosis, nontuberculous mycobacterial infection, lung abscess, pulmonary nocardiosis, or pertussis.
An exposure and social history can focus the differential. For example, coccidioidomycosis or histoplasmosis may present indolently, but have distinct geographic distributions. Bird fanciers may acquire hypersensitivity pneumonitis, psittacosis, or cryptococcosis. Risk factors including smoking history, corticosteroid use, uncontrolled diabetes, and ill contacts should be assessed.
He was discharged from the ED twice in the last 2 weeks after presenting with similar symptoms. On each occasion, he was treated for presumed COPD exacerbations with nebulized albuterol and ipratropium, methylprednisolone followed by oral prednisone, and azithromycin, which did not lead to improvement. Over the last 3 days, he developed lower extremity edema, orthopnea, and dyspnea at rest. He reported worsening fatigue, night sweats, and anorexia. He denied any sick contacts.
Two diagnostic issues have emerged. His edema, orthopnea, and dyspnea at rest suggest a new cause of hypervolemia, perhaps caused by sodium retention from corticosteroids, pulmonary edema from valvular or myocardial disease, or renal failure. More concerning is that he has been treated with azithromycin twice recently but still has night sweats, fatigue, and anorexia. The presence of weight loss despite extracellular volume accumulation suggests an indolent systemic illness. Infection with macrolide-resistant organisms, such as nocardia, mycobacteria, or endemic mycoses, remains high on the differential diagnosis.
His past medical history included hypertension, untreated chronic hepatitis C, tobacco dependence, alcohol use disorder, and extraction of 8 decayed teeth 2 months earlier. He served in a noncombat role during the Vietnam War. He consumed 12 beers weekly with a remote history of alcoholism which required rehabilitation, reported a 50 pack-year smoking history, and denied intravenous (IV) drug use. He lived with an appropriately vaccinated dog and denied recent insect or animal exposures. He had a cat that passed away from an unknown illness 3 years prior. He was in a monogamous relationship with his girlfriend of 35 years. His father had coronary disease. His medications included glyburide, hydrochlorothiazide, lisinopril, theophylline, and meloxicam. Chronic cough, weight loss, diabetes, alcoholism, and history of dental disease raise concern for lung abscess. Oral microbiota such as Streptococcus viridans and Actinomycetes are usually harmless, but when aspirated repeatedly, such as during alcohol intoxication, may evolve into a lung abscess via bronchogenic spread. The combination of unintentional weight loss and smoking history raises concern for lung malignancy. Small cell lung cancer can present with paraneoplastic Cushing’s syndrome and could explain the patient’s volume overload. Finally, human immunodeficiency virus (HIV) serostatus should be determined in all adult patients.
His temperature was 37 °C, blood pressure 161/69 mm Hg, pulse 104 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation was 95% on room air. On examination, he was an unkempt, ill-appearing man. He had poor dentition, but no oral ulcers or petechiae. Pulmonary exam revealed diffuse rhonchi and scattered wheezes. He developed dyspnea after speaking 2 sentences. Cardiovascular exam showed regular tachycardia, normal S1 and S2 heart sounds, and both an S3 and S4 gallop. A grade III/VI holosystolic murmur at the left lower sternal border with apical radiation, and an early, grade III/IV diastolic murmur at the right upper sternal border were present. Neck exam showed jugular venous distention (JVD) 8 cm above the right clavicle. Lower extremities showed symmetric 3+ pitting edema to the knees. His abdomen was soft, nondistended, and without hepatosplenomegaly. There was no lymphadenopathy. Skin exam showed small, healed excoriations on his anterior shins, forearms, and knuckles. There were no petechiae, Janeway lesions, or Osler’s nodes.
These exam findings change the differential substantially. New regurgitant murmurs strongly suggest infective endocarditis (IE). A diastolic murmur is never normal and suggests aortic regurgitation. The holosystolic murmur with apical radiation suggests mitral regurgitation. Cutaneous stigmata should always be sought, but are found in fewer than half of cases of subacute IE, and their absence does not rule out this diagnosis. Disheveled hygiene and excoriations suggest a skin source of infection, and poor dentition is concerning for an oral source. For the moment, the source does not matter. His clinical condition is serious: tachycardia, JVD, edema, and two-sentence dyspnea indicate congestive heart failure. Even before labs and imaging return, inpatient admission is warranted.
Serum sodium concentration was 140 mEq/L, potassium 3.7 mEq/L, chloride 103 mEq/L, bicarbonate 30 mEq/L, blood urea nitrogen (BUN) 26 mg/dL, creatinine 0.8 mg/dL, glucose 120 mg/dL, and calcium 9.0 mg/dL. The white blood cell count was 7100/µL, hemoglobin 11.8 g/dL, and platelet count 101 K/µL. Brain natriuretic peptide (BNP) was 785 pg/mL (reference range 0-100 pg/mL), aspartate aminotransferase 77 U/L, alanine aminotransferase 57 U/L, alkaline phosphatase 125 U/L, total bilirubin 0.8 mg/dL, total protein 7.7 g/dL, and albumin 3.7 g/dL. Erythrocyte sedimentation (ESR) rate was 38 mm/hour (reference range 0-25 mm/hour) and C-reactive protein (CRP) 0.62 mg/dL (reference range <1.0 mg/dL). Cardiac troponins were 0.03 ng/mL (reference range <0.04 ng/mL). Screening for HIV was negative. Urinalysis showed trace blood by dipstick, but no glucose, protein, dysmorphic red blood cells, or casts. Two sets of peripheral blood cultures were drawn. Two sets of blood cultures from his previous ED visits were negative (drawn 6 and 14 days prior).
These laboratory values are nonspecific, and the differential remains unchanged, with top concern for IE, then lung abscess. Ideally, 3 sets of cultures drawn greater than 12 hours apart should be obtained because the likelihood of pathogen detection rises with the volume of blood tested. Thrombocytopenia and microscopic hematuria suggest microangiopathic hemolytic anemia, and a peripheral blood smear should be examined for schistocytes. Glomerulonephritis from immune complex deposition can occur in IE, but is unlikely with a normal serum creatinine and lack of proteinuria, dysmorphic red blood cells, or casts. The elevated BNP suggests cardiac strain due to a regurgitant valve. ESR and CRP are rarely helpful in this situation, and perhaps previous treatment with azithromycin and steroids prevented significant elevation.
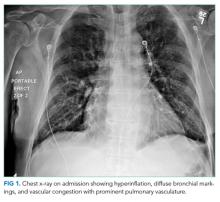
His chest x-ray is not consistent with acute or chronic pulmonary infection. His symptoms, EKG, edema, and improvement with diuresis support the diagnosis of congestive heart failure. The leading diagnosis is left-sided IE, and antimicrobial therapy should not be delayed for the sake of awaiting positive blood cultures. He should immediately receive empiric antibiotics to cover gram-positive bacteria (Methicillin-resistant Staphylococcus aureus, Methicillin-sensitive S. aureus, coagulase-negative staphylococci, and enterococci) and Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella species, and Kingella kingae (the HACEK group). In accordance with Infectious Diseases Society of America (IDSA) practice guidelines, he should empirically receive IV vancomycin plus ceftriaxone and urgently undergo echocardiography.
Transthoracic echocardiogram (TTE) showed severe aortic insufficiency, aortic valve vegetations, and raised suspicion for a moderate-sized vegetation on the anterior leaflet of the mitral valve. There was moderate mitral insufficiency, moderate tricuspid insufficiency, and an elevated right ventricular systolic pressure of 50 mm Hg. The left ventricle showed concentric hypertrophy with an ejection fraction of 55%. A previous echocardiogram 2 years prior showed mild mitral insufficiency, but no aneurysm or aortic insufficiency. Blood cultures from admission yielded no growth.