Pulmonary Perspectives® New Technology Enhances Electromagnetic Navigation Bronchoscopy
Following the National Lung Screening Trial (NLST), which showed at-risk patients screened with CT scans had reduced lung cancer-specific mortality, many institutions have incorporated lung cancer screening protocols into clinical practice (Aberle et al. N Engl J Med. 2011;365[5]:395). These protocols, along with new generation, high resolution multidetector CT scans, have increased the number of detected peripheral lung nodules, many smaller in size. It is estimated that over 150,000 solitary nodules are diagnosed each year in the United States (Herth et al. Expert Rev Respir Med. 2016; 0[8]:901) and, in keeping with the NLST, greater than 25% of subjects screened have lung nodules suspicious for lung cancer. As a result, many leading health practices have created specific lung nodule programs to handle the volume in an effort to deliver timely care in the evaluation of lung cancer.
Pulmonary specialists managing patients with lung nodules are faced with the difficult challenge of deciding if a patient with a nodule is a candidate for serial surveillance, tissue biopsy (transthoracic needle aspiration [TTNA] vs. bronchoscopic biopsy [TBX]), or surgical resection. Calculation of the probability of a nodule being malignant is most helpful in making these decisions for patients with low and high malignancy risk factors, as surveillance and resection are appropriate steps, respectively. However, for those with an intermediate (5%-65%) probability of having a malignant nodule, the diagnostic procedure risks, yields, and timing have to be considered because delayed sampling or false-negative results may negatively impact survival. Kanashiki et al (Oncol Rep. 2003;10[3]:649) showed that worse survival is associated with patients with imaging-to-diagnosis times of greater than 4 months. Over the past decade, image-guided bronchoscopy has been used to improve the yield for tissue sampling of smaller peripheral nodules in a timely fashion. The most common method of image-guided bronchoscopy today is electromagnetic navigation bronchoscopy (ENB).
Electromagnetic navigation bronchoscopy has shown promise for increasing diagnostic yields for peripheral nodules (PN) over conventional bronchoscopy. Over time, the improved yields have plateaued as ENB use in clinical practice increased and limitations of the early generation technology became apparent. Earlier ENB technology uses a single inspiratory CT scan of the chest to reconstruct a 3D virtual model of the airways and parenchyma. A tracked sensor is then used to navigate through the imaging reconstructed airways toward the targeted lesion, the sensor is then removed, and through a dedicated catheter instruments are used to obtain samples from the lesion. In a meta-analysis using this technology, lesions greater than 2 cm had a diagnostic yield ranging from 66.7% to 94.7%. However, as the PN size decreased to less than or equal to 2 cm, the diagnostic yield range dropped significantly with some yields reported as low as 18.2% (van ‘t Westeinde et al. Chest. 2012;142(2):377). More recently, Ost and colleagues performed a multicenter study of consecutive patients undergoing bronchoscopic sampling of PN (Ost et al. Am J Respir Crit Care Med. 2016;193[1]:68). Although it was not a randomized trial and each bronchoscopist influenced the selection of the sampling technique, the authors reported that the diagnostic yields for navigation-guided bronchoscopy were lower than conventional bronchoscopy, 38.5% and 63.7%, respectively. Taken on face value alone, one might conclude that ENB not be used to biopsy PNs. However, deeper analysis of the data showed that 97% of the ENB procedures were performed using the earlier technology described above, suggesting that the single inspiratory imaging CT scan and navigation procedure technique, which differs significantly from conventional bronchoscopy, may have some influence on the lower than expected yields.
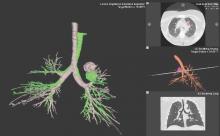
Despite increasing use and experience with ENB, diagnostic yields remain static. Chen and colleagues hypothesized that using a single inspiratory CT scan may not allow the endoscopist to make adjustments for PN movement as the lung moves during the respiratory cycle. Using different imaging protocol, the investigators assessed movement of 85 lung nodules during the respiratory cycle with paired-full inspiration and tidal-volume expiration, thin sliced (0.5-1.0 mm) CT scans. They found that the average motion of all lesions during respiration was 17.6 mm, 12.2 mm in the right-upper lobe, 10.6 mm in the left-upper lobe, and 25.3 mm and 23.8 mm in the right- and left- lower lobes, respectively (Chen et al. Chest. 2015;147[5];1275) (Fig. 1). They concluded that the location of targeted lesions on a single inspiration planning CT scan alone does not accurately represent the position of the lesion during bronchoscopy.
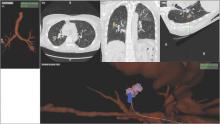
Although being able to correct for nodule movement throughout the respiratory cycle during the procedure is a significant improvement, it doesn’t guarantee that the tissue sample is obtained from the targeted lesion. To accomplish that, the system would have to be able to determine when the instrument being used to sample is in the target. The earlier ENB systems allowed for navigation to the target with a separate sensor through a steerable catheter. However, when the target was reached, the sensor had to be removed so that sampling instruments could be introduced into the catheter. Since the instruments are not tracked and the movement of the nodule is occurring, there is no guarantee that the instrument is in the target at the time of sampling. Advanced technology now allows for the tracking sensor to be placed in the tip of standard bronchoscopy instruments, making them “tip-tracked” and able to be used with standard bronchoscopes and equipment; thus, making the new ENB procedure similar to conventional bronchoscopy that was shown to have higher diagnostic yields (Figs. 2 and 3).