Obesity and lung disease in the era of GLP-1 agonists
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
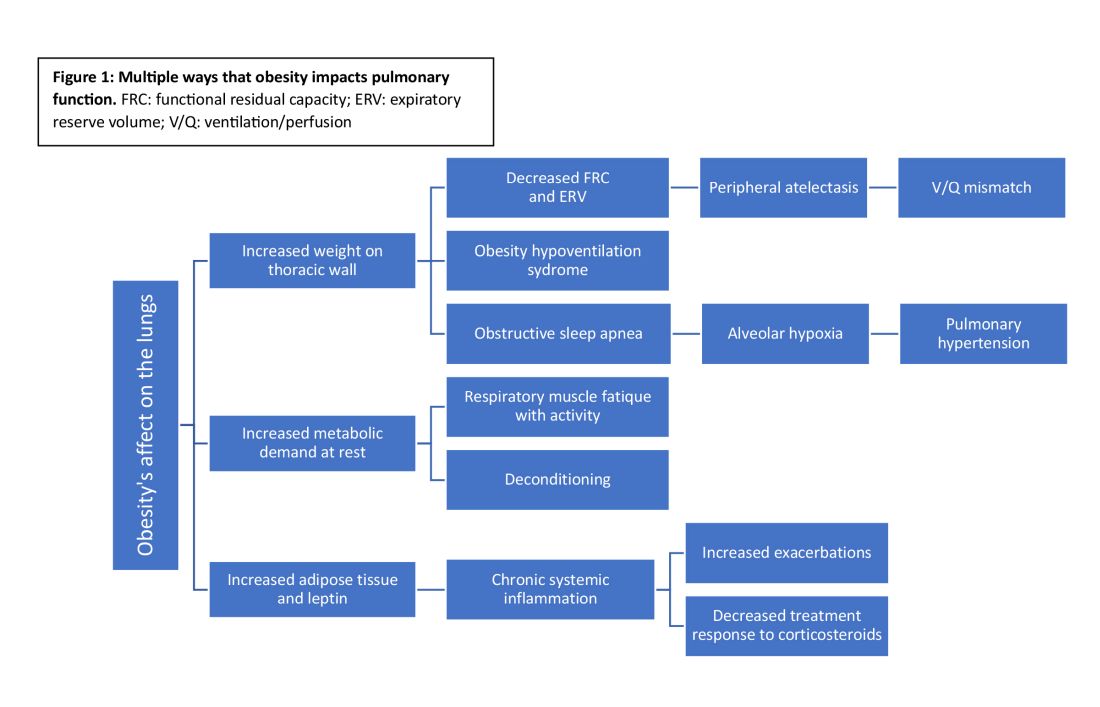
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.








