Chronic lymphocytic leukemia and apparent hyperkalemia
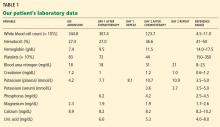
A 64-year-old man with chronic lymphocytic leukemia (CLL), Rai stage IV, was admitted to the hospital to undergo his first cycle of chemotherapy with fludarabine, cyclophosphamide, and rituximab. The physical examination at this time was normal except for splenomegaly and painless bilateral inguinal lymphadenopathy. His laboratory results on admission are shown in Table 1.
On the first day after chemotherapy, laboratory testing revealed an elevated plasma potassium level of 7.7 mmol/L. The specimen was drawn into a BD Vacutainer plasma separator tube with lithium-heparin additive (Becton, Dickinson, and Company, Franklin Lake, NJ) and analyzed on a Unicel DXC 800 chemistry analyzer (Beckman Coulter, Inc, Brea, CA).
1. Which electrocardiographic feature is not associated with hyperkalemia?
- Peaked P waves
- Prolonged PR interval
- Shortened QT interval
- Widened QRS
- Asystole
EVALUATING CARDIAC TOXICITY FROM HYPERKALEMIA
Hyperkalemia is not associated with peaked P waves, but rather with a reduction in the size of the P waves.
Hyperkalemia, defined as a plasma potassium concentration above 5.5 mmol/L, occurs as a result either of a release or a shift of intracellular potassium into the intravascular space or of decreased renal excretion. The earliest changes noted on electrocardiography are peaking and narrowing of T waves, followed by shortening of the QT interval. As hyperkalemia progresses, electrocardiography may show bradycardia, absent P waves, and PR interval prolongation, including second- or third-degree atrioventricular block.
At a plasma potassium concentration greater than 7 mmol/L, there may be a junctional escape rhythm, a sine wave pattern with widening of the QRS complex merging with T waves, ventricular fibrillation, or asystole. However, even with high potassium levels, electrocardiographic changes may be absent.1
The patient denied fatigue, muscle weakness, or palpitations. Electrocardiography did not show peaked T waves, shortened QT intervals, decreased P waves, prolonged PR interval, or widening of the QRS interval.
2. Which is an appropriate intervention for hyperkalemia with cardiac toxicity?
- Membrane stabilization with calcium gluconate
- Shifting potassium into the cells with a beta-adrenergic agonist given by nebulization
- Shifting potassium into the cells with insulin and dextrose
- Removal of potassium with sodium polystyrene sulfonate (Kayexalate)
- Removal of potassium with dialysis
In the setting of cardiac toxicity, the management of hyperkalemia involves stabilizing the cardiac muscle membrane, shifting potassium into the cells, and removing potassium from the body. It is important to do all three interventions when cardiac toxicity is present to provide sustained therapeutic benefit.2
MANAGING HYPERKALEMIA
General principles
When potassium levels are above 6 mmol/L, electrocardiography should always be done. Immediately stop potassium supplementation and any drugs that can cause hyperkalemia, such as potassium-sparing diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, nonsteroidal anti-inflammatory drugs, and trimethoprim-sulfamethoxazole (Bactrim). If the potassium level is greater than 6.5 mmol/L, the patient should be placed on telemetric monitoring, and the potassium level should be measured often.
Hyperkalemia with cardiac toxicity
Membrane stabilization involves intravenous infusion of 10 mL of 10% calcium gluconate. The onset of action is 1 to 3 minutes, and the duration of action is 30 to 60 minutes.
Shifting of potassium is done either with insulin or with a beta-adrenergic agonist nebulizer. With insulin, 10 U of regular insulin is given intravenously along with 50 mL of 50% dextrose; the onset of action is 20 minutes, and the duration is 4 to 6 hours. The dose of beta-adrenergic agonist depends on the type used; the onset of action is 20 minutes, with a duration of 2 to 4 hours.
Removal of potassium is achieved either by drug therapy or by dialysis. Sodium or calcium polystyrene sulfonate is given by mouth, 15 g every 6 hours, or 30 to 60 g by retention enema; the onset of action is 1 to 2 hours, with a duration of 4 to 6 hours. If dialysis is used, 2 to 3 hours is recommended; the onset of action is immediate and lasts for the duration of the dialysis session.
CASE CONTINUED
The patient was moved to a telemetry unit. A repeat plasma potassium measurement after 30 minutes confirmed hyperkalemia (Table 1). The specimen was transported to the laboratory via a pneumatic tube system, centrifuged at 3,300 rpm for 10 minutes, and analyzed on a Beckman Unicel DXC 800 chemistry analyzer. The time from specimen collection to analysis was approximately 60 minutes.
He was treated with oral sodium polystyrene sulfonate because no electrocardiographic changes were observed. Subsequent plasma potassium levels drawn, collected, and analyzed with the same technique described above were persistently high. Repeated electrocardiography continued to show no changes related to hyperkalemia.