Personalized targeted therapy in advanced non–small cell lung cancer
ABSTRACTPersonalized targeted therapy for advanced non–small cell lung cancer (NSCLC) primarily relies on the concept of “oncogene addiction,” in which multiple genetic abnormalities are addicted to one or a few genes for tumor cell maintenance and survival. Several molecular aberrations have been identified in NSCLC, with subsequent development of drugs targeted to these aberrations; gefitinib, erlotinib, and cetuximab for the treatment of NSCLC harboring epidermal growth factor receptor mutation or overexpression, and crizotinib for the treatment of NSCLC with the EML4-ALK fusion translocation oncogene being some examples. A more recent actionable target is MET, a multifaceted receptor tyrosine kinase within the human kinome. Cellular heterogeneity within an oncogene-addicted tumor can cause resistance to targeted therapy after an initial response. As our understanding of tumor heterogeneity and tumor resistance mechanisms evolves, more rational therapies and combinations of therapies can be expected.
The efficacy of therapy targeted to a specific oncogene is convincing evidence of “oncogene addiction,” or the concept that some cancers rely on or are “addicted to” a specific gene for their survival and proliferation. In the case of non–small cell lung cancer (NSCLC), drugs that target epidermal growth factor receptor (EGFR) have been proven more effective than conventional chemotherapy in patients with sensitizing EGFR mutations.1
Lung cancer oncogenes can drive oncogenic signaling pathways within tumor cells. Activation of EGFR signaling that drives cell proliferation through pathways such as RAS/RAF/MEK/ERK and the cell survival pathways P13K and AKT has been demonstrated in never-smokers. In heavy smokers, KRAS oncogene mutation is the dominant promoter of activation of oncogenic signaling pathways; it predicts a poor prognosis (especially for lung adenocarcinoma), and it is essentially mutually exclusive with EGFR mutations.
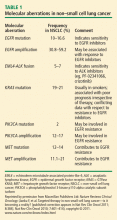
More than 50% of cases of NSCLC have known oncogene mutations for which targeted therapeutics are available.2 For example, gefitinib and erlotinib are the effective inhibitors for the EGFR oncogene mutation, sunitinib for platelet-derived growth factor receptor (PDGFR) amplification, and lapatinib for the less common ERBB2 insertion.
- EGFR mutations and amplifications
- EML4-ALK translocation fusions
- KRAS mutations
- PIK3CA mutations
- MET mutations, alternative splicing, amplification, and overexpression
PLATINUM DOUBLET AS STANDARD
Multiple platinum-based combinations of chemotherapy are in use as first-line therapy for advanced NSCLC. An overall survival (OS) benefit has been established with the use of doublet regimens, but no platinum-based doublet regimen has been proven superior to another on the end point of OS in clinical trials.4
Adding a third agent increases the response rate in advanced NSCLC but does not improve OS; the exception is bevacizumab, a monoclonal antibody targeted to vascular endothelial growth factor (VEGF). Sandler et al5 demonstrated a survival benefit when bevacizumab was added to paclitaxel-carboplatin in a recent study that led to US Food and Drug Administration (FDA) approval of bevacizumab for the treatment of NSCLC.
The next oncogene target explored in advanced NSCLC was EGFR. The tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib, lapatinib, and the monoclonal antibody cetuximab are all clinical inhibitory agents targeting EGFR.
Previously treated NSCLC
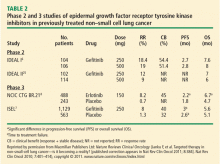
In the phase 3 National Cancer Institute of Canada (NCIC) Clinical Trials Group (CTG) BR.21 trial, erlotinib demonstrated significant superiority over placebo as second- or third-line chemotherapy on PFS and OS in unselected patients with NSCLC. The results led to its approval as treatment for advanced NSCLC in patients who have received at least one prior chemotherapy regimen.8
Patient survival in NCIC CTG BR. 21 was evaluated in a series of patient subsets in exploratory univariate analyses. The effect of erlotinib on survival was similar across most subsets. A greater effect on survival by erlotinib was observed in patients who had never smoked (hazard ratio [HR] = 0.42). In the subgroup of patients who never smoked, EGFR status was predictive of erlotinib survival benefit. Patients who never smoked and were EGFR-positive had a survival benefit with erlotinib (HR = 0.27). There were too few EGFR-negative patients who never smoked to reach a conclusion.