The role of adjuvant chemotherapy in early-stage and locally advanced non–small cell lung cancer
ABSTRACTAdjuvant chemotherapy benefits only a small proportion of patients in the setting of resected early-stage non–small cell lung cancer, and in unselected patients, any benefit is modest. Analysis of clinical trials of adjuvant chemotherapy revealed that differential expression of DNA repair proteins and a 15-gene expression profile affected outcomes with treatment. Biomarkers and gene expression profiles are now being studied in prospective clinical trials to gauge their value in selection of adjuvant therapy and individualization of therapy.
Despite surgery, 40% to 75% of patients with stage I to IIIA non–small cell lung cancer (NSCLC) will die within 5 years. After multiple trials showed no survival advantage to chemotherapy in the adjuvant setting for the treatment of locally advanced NSCLC, the first hint of benefit came in 1995 with the publication of a meta-analysis of 14 clinical trials, which showed a nonsignificant 5% improvement in 5-year survival with chemotherapy after surgery.1
A second meta-analysis, this one conducted by the Lung Adjuvant Cisplatin Evaluation (LACE) Collaborative Group, demonstrated a hazard ratio (HR) of 0.89 (P = .005) on the end point of overall survival with the use of postoperative cisplatin-based chemotherapy in patients with NSCLC; this translates to a 5-year absolute improvement of 5.4% from chemotherapy.2 The survival benefit was confined to patients with stage II and stage III disease.
Post hoc exploratory subgroup analyses of the Cancer and Leukemia Group B (CALGB) 96333 and Adjuvant Navelbine International Trialist Association (ANITA)4 trials revealed a significant survival benefit to four cycles of cisplatin-based adjuvant chemotherapy in patients with stage Ib disease who had tumors 4 cm or larger.
BIOMARKERS
Prognostic and predictive biomarkers beyond cancer stage are needed, as only 10% to 15% of patients with resected NSCLC who receive chemotherapy derive a benefit. Predictive markers can be used to guide therapeutic decision-making, and prognostic markers permit estimation of patient outcome independent of treatment modality.
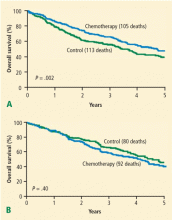
As with ERCC1, expression of the DNA mismatch repair protein mutS homolog 2 (MSH2)7 is both prognostic and predictive after surgery. In a separate biomarker analysis from the IALT study, approximately two-thirds of patients with NSCLC had MSH2-negative tumors by immunohistochemistry, indicating lack of expression of MSH2 in tumors. Patients with expression of MSH2, who have intact mismatch repair, had a better prognosis and benefitted less from systemic chemotherapy than those with an absence of MSH2 expression.8
Individually, ERCC1 and MSH2 have similar power in predicting benefit from adjuvant chemotherapy in NSCLC; the HR for death was similar in patients with low expression of either gene.8 The two biomarkers combined, however, were more powerful than either alone in their ability to predict a survival advantage with chemotherapy.8 In an evaluation of 658 patients with NSCLC for whom both biomarkers were available, patients who expressed low tumor levels of both ERCC1 and MSH2 had an HR for death that was 35% lower with adjuvant chemotherapy compared with surgery alone after median follow-up of 7.5 years; the presence of two positive biomarkers was associated with an increase in the HR for death by 32%. Validation of these findings in a phase 3 setting will be necessary before these biomarkers can be used in the clinical setting.
The Southwest Oncology Group (SWOG) is conducting a trial (SWOG 0720) in patients with stage I NSCLC to determine whether a subset based on ERCC1 and ribonucleotide reductase M1 (RRM1) status will derive benefit from adjuvant therapy with gemcitabine together with cisplatin. Ribonucleotide reductase subunit 1 is the regulatory subunit of ribonuclease reductase, which is an enzyme that catalyzes the deoxynucleotide production required for DNA repair.
Two other clinical trials, under way but not completed, testing various forms of chemotherapy and targeted therapy based on ERCC1 and epidermal growth factor receptor (EGFR) mutation status are the Tailored Post-Surgical Therapy in Early Stage NSCLC (TASTE) and the International Tailored Chemotherapy Adjuvant (ITACA) trials. The TASTE trial is comparing standard chemotherapy (cisplatin plus pemetrexed) with customized adjuvant treatment based on EGFR and ERCC1 status in patients with stage II or IIIa nonsquamous NSCLC. The ITACA trial is a phase 3 study of pemetrexed, cisplatin, and radiotherapy determined by thymidylate synthase (TS) and ERCC1 gene expression levels in patients with stage II to III completely resected NSCLC. TS is an enzyme responsible for maintaining intracellular levels of thymidine, important for DNA synthesis and repair, and may serve as a predictor of response to pemetrexed.