Expanding indications for TAVR: The preferred procedure in intermediate-risk patients?
ABSTRACT
Transcatheter aortic valve replacement (TAVR) has steadily replaced surgical aortic valve replacement (SAVR) in symptomatic patients with severe aortic stenosis, primarily those at high risk for surgical complications. As TAVR use increases, spurred by technological advances in valve design and patient preferences for the less-invasive procedure, studies have provided data supporting the efficacy and safety of TAVR. Recently, TAVR has expanded to intermediate-risk patients, increasing the potential patient population. Although emerging evidence supports its use in lower-risk patients, some adverse events may limit its adoption in a wider patient population. These include stroke, paravalvular leak, valve durability, valve thrombosis, and need for pacemaker replacement. Ongoing clinical trials are expected to provide answers.
KEY POINTS
- TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
- The US Food and Drug Administration has approved TAVR with open-heart surgery.
- Initial outcomes support expanding TAVR to intermediate-risk patients, including mortality and stroke data, but concerns exist related to valve durability, valve thrombosis, and rates of permanent pacemaker implantation.
Surgical aortic valve replacement (SAVR) started in the 1960s with a porcine aortic valve sutured to a stainless steel frame. The first human transcatheter aortic valve replacement (TAVR) procedure in the United States was in 2002. In the past 15 years, technological advances in heart valve design have made TAVR the preferred alternative in patients at high risk for surgical complications. This article outlines studies comparing balloon-expandable TAVR vs SAVR for patients at extreme, high, and intermediate surgical risk, and presents evidence that supports the expanded use of TAVR in patients at lower surgical risk.
TAVR: THE PREFERRED ALTERNATIVE TO SURGERY
Investigators next established TAVR outcomes as being noninferior to SAVR in high surgical risk patients (PARTNER trial cohort A) at 1 year.2 A midterm follow-up of this study published in 2015 reported comparable rates of all-cause mortality at 5 years in high-risk patients undergoing TAVR vs SAVR, thus confirming the noninferiority of TAVR vs a surgical approach in high-risk patients for the longest duration of follow-up currently available.3
For patients, if the results of 2 different procedures are similar, they are typically going to choose the less invasive option. As a result, use of TAVR has increased: nearly 300,000 procedures have been performed worldwide, and approximately 75,000 were completed in 2016 alone. These numbers are projected to increase fourfold in the next 10 years. In the United States, almost one-third of Medicare-reported aortic valve replacements in 2015 were performed using TAVR.4
These data show that TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
TAVR IN INTERMEDIATE-RISK PATIENTS
The US Food and Drug Administration (FDA) initially approved TAVR for patients judged to be ineligible for open-chest valve replacement cardiac surgery or at high risk for SAVR. This represents a small percentage of the total patient population needing aortic valve replacement. The Society of Thoracic Surgeons database of aortic valve disease cases during 2002 to 2010 (N = 141,905) shows that just 6.2% were ranked as high risk (ie, population eligible for TAVR in 2016). Most patients (79.9%) were low risk, and 13.9% were intermediate risk.5
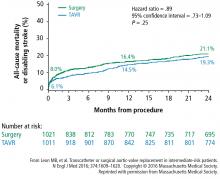
A subanalysis of the transfemoral-access cohort provided additional support for TAVR. It showed that the rate of death and stroke in this cohort began to trend more favorably for TAVR. At 24 months, the difference in the primary end point was statistically significant in favor of TAVR (16.3% vs 20.0% for surgery; P = .04).1
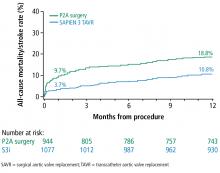
Based on these data, in August 2016, the FDA approved the Sapien valves for use in patients with aortic valve stenosis who are at intermediate risk of death or complications associated with open-heart surgery. If the differences in outcomes reported during the PARTNER S3i trial are extrapolated to the total number of valve replacement surgeries performed worldwide, the potential number of patients who may benefit from TAVR is substantial.