Cardiac amyloidosis: An update on diagnosis and treatment
ABSTRACT
Cardiac amyloidosis (CA), once thought to be a rare disease, is increasingly recognized due to enhanced clinical awareness and better diagnostic imaging. CA is becoming of heightened interest to the cardiology community given more effective treatment strategies for light chain amyloidosis (AL), as well as emerging therapies for transthyretin amyloidosis (ATTR). Furthermore, reversing amyloid deposition in affected organs using monoclonal antibodies is actively being tested in clinical trials. A high index of suspicion and a systematic approach to the diagnosis of CA can lead to referral to a center of expertise for timely treatment.
KEY POINTS
- AL and ATTR are the 2 main types of amyloidosis that affect the heart.
- Serum and urine protein electrophoresis are inadequate laboratory tests to screen for AL given low sensitivity, and should be replaced by the serum free light chain assay as well as immunofixation of the serum and urine.
- AL cardiac amyloidosis (AL-CA) requires timely diagnosis and referral to hematology due to high mortality without prompt treatment.
- 99mTechnetium pyrophosphate bone scintigraphy is an affordable, noninvasive tool that has revolutionized the diagnosis of ATTR cardiac amyloidosis (ATTR-CA).
- The US Food and Drug Administration will likely approve new therapies for ATTR in late 2018.
WHAT IS AMYLOIDOSIS?
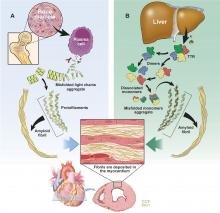
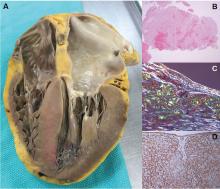
Amyloidosis is a protein deposition disease in which a specific precursor protein pathologically misfolds from its physiologic tertiary structure into a more linear shape dominated by beta-pleated sheets. The misfolded protein aggregates into oligomers, eventually forming insoluble amyloid fibrils that deposit extracellularly in tissues. Both the circulating oligomers, which are cytotoxic, and the fibrils, which cause distortion of the tissue architecture, lead to organ dysfunction. Amyloid fibrils are rigid, nonbranching structures, 7 to 10 nanometers in diameter, with a characteristic appearance on electron microscopy. Affinity for Congo red staining, which binds to the beta-pleated sheets, produces the pathognomonic “apple-green” birefringence when visualized under polarized light microscopy. Universal to all amyloid fibrils are chaperone proteins such as serum amyloid P (SAP) and glycosaminoglycans, as well as calcium. There are more than 30 different precursor proteins implicated in various amyloid diseases, arising as hereditary or nonhereditary, localized or systemic, with different organ involvement and prognosis.1–3
TWO MAIN TYPES OF CARDIAC AMYLOIDOSIS
Light chain amyloidosis (AL)
AL, formerly called primary amyloidosis, is a clonal plasma cell disorder due to the overproduction and misfolding of antibody light chain fragments. It is a rare disease with about 3,000 new cases per year in the United States.5 The median age at diagnosis is 63, although it can present in patients in their 30s and 40s.5,6 It is a systemic disease that often affects the heart, but it can affect several other organs, most commonly the kidneys, gastrointestinal (GI) tract, and nervous system.7
AL is a more aggressive disease than ATTR, with a median untreated survival of less than 6 months in patients who present with heart failure.8 Early diagnosis is crucial as mortality is high without prompt treatment.
Transthyretin amyloidosis (ATTR)
ATTR is due to misfolding of the liver-derived precursor protein transthyretin (TTR) (previously called prealbumin), either as an acquired wild-type variant (ATTRwt) or as a hereditary mutant variant (ATTRm). ATTRwt, known previously as senile CA, typically affects older males and presents as a late onset hypertrophic restrictive cardiomyopathy, often preceded by carpal tunnel syndrome or spinal stenosis or both. The ATTRm variant, caused by one of many different point mutations in the TTR gene, can manifest as a polyneuropathy, cardiomyopathy, or a mixed phenotype that varies according to the specific mutation.
While ATTR portends a better prognosis than AL, it is still a progressive disorder with significantly reduced survival and quality of life. The median survival of patients with the ATTRwt variant is about 4 years and for patients with the ATTRm variant, survival depends on the mutation.9 TTR is a protein tetramer composed of 4 identical 127-amino acid monomers noncovalently bound at a dimer-dimer interface (Figure 1). It is a transport protein for thyroxine and retinol binding protein. The dissociation of the tetramer is the rate-limiting step for amyloid fibrillogenesis. Differentiating the ATTRwt variant and the ATTRm variant is done by testing the TTR gene for a mutation.1,3
How common is the ATTRwt variant? The ATTRwt variant is often an unrecognized cause of diastolic heart failure in the elderly, with up to 25% of patients 85 and older showing ATTRwt amyloid deposits on autopsy studies.10 A recent study showed that 13% of patients 60 and older hospitalized with heart failure with preserved ejection fraction had grade 2 to 3 uptake on 99mtechnetium-pyrophosphate (99mTcPYP) scintigraphy, which is consistent with ATTR-CA.11 In 43 consecutive patients undergoing transcatheter aortic valve replacement, 11.6% were found to have significant uptake on 99mTcPYP scan.12 It is clear given the aging population that the ATTRwt variant will become the most common form of amyloidosis. It is much more common in white males, with a median age at diagnosis of 75.13 Carpal tunnel syndrome (almost always bilateral) and spinal stenosis are present in about 50% of patients diagnosed with ATTRwt-CA and often precede clinical presentation of heart failure by 5 to 15 years.14–17
How common is the ATTRm variant? There are more than 100 point mutations in the TTR gene that lead to various familial TTR-related amyloid syndromes, either neuropathic (familial amyloid polyneuropathy [FAP]) or cardiomyopathic (familial amyloid cardiomyopathy).18 The most common mutation in the United States is V122I in which there is an isoleucine substitution for valine at the 122nd amino acid position. This mutation is seen in African Americans, 3% to 4% of whom are heterozygote carriers.19 Although the true penetrance is unknown, this mutation can lead to a late-onset restrictive cardiomyopathy with minimal neuropathy and is frequently misdiagnosed as hypertensive heart disease or diastolic heart failure. The median survival for V122I ATTRm-CA is about 2 years but likely depends on the stage at the time of diagnosis.20 The second most common mutation in the United States, T60A, is seen in patients of Irish descent and causes a mixed neuropathy and cardiomyopathy.17
PATHOLOGY AND PATHOPHYSIOLOGY OF CA
The atria are universally involved with interatrial septal thickening, which can lead to poor atrial function and increased rates of atrial fibrillation (ATTR more so than AL).21,29 The conduction system can be affected causing varying degrees of heart block, as well as bundle branch block (ATTR more so than AL).30 The valves are usually thickened, often associated with mild to moderate regurgitation. Pericardial involvement can lead to small pericardial effusions (large effusions are rare), and coronary involvement (classically, small intramural vessels) can lead to ischemia and angina with normal epicardial coronaries (AL more so than ATTR).31–33
Thickened left and right ventricular walls result in a nondilated ventricle that is stiff and poorly compliant, resulting in progressive diastolic filling abnormalities. Systolic dysfunction can be seen in severe and advanced disease. Importantly, ejection fraction measured by echocardiography is misleading in CA, as reduced end-diastolic volume produces a low stroke volume. For example, an ejection fraction of 50%, when starting at a significantly reduced end-diastolic volume (for example, 70 mL), leads to a significantly reduced stroke volume (35 mL) and, thus, cardiac output. This explains why patients with CA cannot usually tolerate reduced heart rates, as their cardiac output is dependent on heart rate.34–36