Selecting a Direct Oral Anticoagulant for the Geriatric Patient with Nonvalvular Atrial Fibrillation
From the Ernest Mario School of Pharmacy, Rutgers University, Piscataway, NJ.
Abstract
- Objective: To provide a clinical summary of the available data evaluating the use of direct oral anticoagulants (DOACs) in geriatric patients with nonvalvular atrial fibrillation.
- Methods: MEDLINE, Web of Science, and Google Scholar were used to identify pertinent systematic reviews, randomized controlled trials, observational studies, and pharmacokinetic studies evaluating use of DOACs in the geriatric population.
- Results: A total of 8 systemic reviews, 5 randomized controlled trials, 2 observational trials, and 5 pharmacokinetic studies of relevance were identified for inclusion in this review. The landscape of anticoagulation has dramatically changed over the past 5 years beginning with the development and marketing of an oral direct thrombin inhibitor and followed by 3 oral direct factor Xa inhibitors. Despite significant advances in this oral anticoagulation arena, many questions remain as to the best therapeutic approach in the geriatric population as the literature is lacking. This population has a higher risk of stroke; however, due to the increased risk of bleeding clinicians may often defer anticoagulant therapy due to the fear of hemorrhagic complications. Clinicians must consider the risk-benefit ratio and the associated outcomes in geriatric patients compared to other patient populations.
- Conclusions: Interpreting the available literature and understanding the benefits and limitations of the DOACs is critical when selecting the most appropriate pharmacologic strategy in geriatric patients.
Anticoagulants are among the top 5 drug classes associated with patient harm in the US [1] and are commonly reported as contributing to hospitalizations [2]. In just one quarter in 2012 alone, warfarin, dabigatran, and rivaroxaban accounted for 1734 of 50,289 adverse events reported to the Food and Drug Administration (FDA), including 233 deaths [3]. Appropriate use of anticoagulant agents and consideration of individual patient risk factors are essential to mitigate the occurrence of adverse consequences, especially in the geriatric population. This population is more likely to have risk factors for adverse drug events, for example, polypharmacy, age-related changes in pharmacokinetics and pharmacodynamics, and diminished organ function (ie, renal and hepatic) [4,5]. Another important consideration is the lack of consensus on the definition of a “geriatric” or “elderly” patient. Although many consider a chronological age of > 65 years as the defining variable for a geriatric individual, this definition does not account for overall health status [6,7]. Clinicians should consider this shortcoming when evaluating the quality of geriatric studies. For example, a study claiming to evaluate the pharmacokinetics of a drug in a geriatric population enrolling healthy subjects aged > 65 years may result in data that do not translate to clinical practice.
Compounding the concern for iatrogenic events is the frequency of anticoagulant use in the geriatric population, as several indications are found more commonly in this age-group. Stroke prevention in nonvalvular atrial fibrillation (AF), the most common arrhythmia in the elderly, is a common indication for long-term anticoagulation [8]. The prevalence of AF increases with age and is usually higher in men than in women [9,10]. AF is generally uncommon before 60 years of age, but the prevalence increases noticeably thereafter, affecting approximately 10% of the overall population by 80 years of age [11]. The median age of patients who have AF is 75 years with approximately 70% of patients between 65 and 85 years of age [8,12]. Currently in the United States, an estimated 2.3 million people are diagnosed with AF [8]. In 2020, the AF population is predicted to increase to 7.5 million individuals with an expected prevalence of 13.5% among individuals ≥ 75 years of age, and 18.2% for those ≥ 85 years of age [13]. These data underscore the importance of considering the influence of age on the balance between efficacy and safety of anticoagulant therapy.
Direct oral anticoagulants (DOACs) represent the first alternatives to warfarin in over 6 decades. Currently available products in US include apixaban, dabigatran, edoxaban, and rivaroxaban. DOACs possess many of the characteristics of an ideal anticoagulant, including predictable pharmacokinetics, a wider therapeutic window compared to warfarin, minimal drug interactions, a fixed dose, and no need for routine evaluation of coagulation parameters. The safety and efficacy of the DOACs for stroke prevention in nonvalvular AF have been substantiated in several landmark clinical trials [14–16]. Yet there are several important questions that need to be addressed, such as management of excessive anticoagulation, clinical outcome data with renally adjusted doses (an exclusion criterion in many landmark studies was a creatinine clearance of < 25–30 mL/min), whether monitoring of coagulation parameters could enhance efficacy and safety, and optimal dosing strategies in geriatric patients. This review provides clinicians a summary of data from landmark studies, post-marketing surveillance, and pharmacokinetic evaluations to support DOAC selection in the geriatric population.
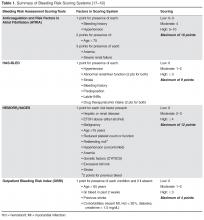
Evaluating Bleeding Risk
These tools have been extensively evaluated with warfarin therapy, but their performance in predicting DOAC-related bleeding has not been definitively established. Nonetheless, until tools evaluated specifically for DOACs are developed, it is reasonable to use these for risk-prediction in combination with clinical judgment. As an example, the European Society of Cardiology guideline on the use of non–vitamin K antagonist (VKA) anticoagulants in patients with nonvalvular AF suggests that the HAS-BLED score may be used to identify risk factors for bleeding and correct those that are modifiable [20]. The HAS-BLED score is validated for VKA and non-VKA anticoagulants (early-generation oral direct thrombin inhibitor ximelgatran) [21] and is the only bleeding risk score predictive for intracranial hemorrhage [19]. In a 2013 “real world” comparison, HAS-BLED was easier to use and had better predictive accuracy that ATRIA [22].
One of the major challenges in geriatric patients is that those at highest risk for bleeding are those who would have the greatest benefit from anticoagulation [23]. The prediction scores can help clinicians balance the risk-benefit ratio for anticoagulation on a case by case basis. Although the scoring systems take into consideration several factors, including medical conditions that have been shown to significantly increase bleeding risk, including hypertension, cerebrovascular disease, ischemic stroke, serious heart disease, diabetes, renal insufficiency, alcoholism and liver disease, not all are included in every scoring scheme [23]. These conditions are more common among elderly patients, and this should be taken into account when estimating the risk-benefit ratio of oral anticoagulation [15]. Patients’ preferences should also be taken into account. It is essential for clinicians to clearly discuss treatment options with patients as data suggest that clinician and patient perceptions of anticoagulation are often mismatched [24–26].
Performance of TSOACS in Landmark Studies
Some specific differences in outcomes seen in landmark studies that may facilitate selection among the DOACs include the risk of major bleeding, risk of gastrointestinal bleeding, risk of acute coronary syndrome, exclusion of valvular heart disease, and noninferiority versus superiority as the primary endpoint when compared to warfarin.
Major Bleeding
Gastrointestinal Bleeding
Among all of the DOACs, gastrointestinal (GI) bleeding was significantly greater with dabigatran, edoxaban, and rivaroxaban when compared to warfarin (HR, 1.49; 95% CI, 1.21–1.84; HR, 1.23; 95% CI, 1.02–1.50; and HR, 1.61; 95% CI, 1.30–1.99, respectively; P < 0.05 for all) [14–16] in landmark studies. Based on these data, clinicians may consider the selection of apixaban in patients with a previous history of GI pathology. GI bleeding may be more common in elderly patients due to the potential for preexisting GI pathology and high local concentrations of drug [29]. Clemens and colleagues suggested an “anticoagulation GI stress test” may predict GI malignancy [33]. They found that patients on DOACs that presented with a GI bleed were more likely to present with a GI malignancy. As such, it is reasonable to screen patients with a fecal occult blood test within the first month after initiating TSOAC treatment and then annually.
Acute Coronary Syndrome
A higher rate of myocardial infarction was observed with dabigatran 150 mg versus warfarin (0.74% vs 0.53% per year; P = 0.048) in the RE-LY study [16]. Whether the increase in myocardial infarction was due to dabigatran as a causative agent or warfarin’s ability to reduce the risk of myocardial infarction to a larger extent compared with dabigatran is unknown. Nonetheless, it may be prudent to use an alternative therapy in patients with a history of acute coronary syndrome.
Valvular Heart Disease
The risk of stroke and systemic embolism is higher in patients with valvular heart disease [34]. Patients with moderate to severe mitral stenosis or mechanical prosthetic heart valves were excluded from the DOAC landmark studies. Dabigatran was evaluated for prevention of stroke and systemic embolism in patients with valvular heart disease in the RE-ALIGN study [35,36]. Patients were randomized to warfarin titrated to a target INR of 2 to 3 or 2.5 to 3.5 on the basis of thromboembolic risk or dabigatran 150 mg, 220 mg, or 300 mg twice daily adjusted to a targeted trough of ≥ 50 ng/mL. The trial was terminated early due to a worse primary outcome (composite of stroke, systemic embolism, myocardial infarction, and death) with dabigatran versus warfarin (HR, 3.37, 95% CI, 0.76–14.95; P = 0.11). In addition, bleeding rates (any bleeding) was significantly greater with dabigatran (27%) versus warfarin (12%) (P = 0.01). Based on these data and the lack of data with the other TSOACs, warfarin remains the standard of care for valvular heart disease [37]. In patients with a previous bioprosthetic valve with AF, patients with mitral insufficiency, or aortic stensosis, TSOACs may be considered [37].
Landmark Study Efficacy Endpoints
The primary endpoint in each of the landmark studies was a composite of stroke (ischemic or hemorrhagic) and systemic embolism. For the primary endpoint only dabigatran 150 mg twice daily and apixaban 5 mg twice daily were found to be superior to warfarin for the prevention of stroke or systemic embolism in nonvalvular AF (HR, 0.66; 95% CI, 0.53–0.82; P < 0.001 and HR, 0.66; 95% CI, 0.66–0.95; P = 0.01, respectively). Both edoxaban (60 mg and 30 mg daily) and rivaroxaban were noninferior to warfarin for the primary endpoint. In terms of ischemic stroke, only dabigatran 150 mg twice daily was superior to warfarin for the reduction in ischemic stroke in patients with nonvalvular AF (HR, 0.76; 95% CI, 0.60–0.98; P = 0.03) [19]. All of the DOACs demonstrated a reduction in hemorrhagic stroke.
TSOAC Use in Elderly Patitents
Pharmacokinetic Evaluations
Several pharmacokinetic studies have evaluated the influence of age on DOAC disposition. In a study evaluating the influence of age on apixaban disposition, the area under the concentration-time curve to infinity was 32% higher in the elderly (aged 65 years or older) compared to the younger subjects (< age 40 years) [38]. These data provide the rationale for dosage adjustment in individuals aged 80 years or older with either low body mass (weight less than or equal to 60 kg) or renal impairment (serum creatinine 1.5 mg/dL or higher). In a pharmacokinetic study evaluating dabigatran in patients > 65 years of age, the time to steady state ranged from 2 to 3 days, correlating to a half-life of 12 to 14 hours, and peak concentrations (256 ng/mL females, 255 ng/mL males) were reached after a median of 3 hours (range, 2.0–4.0 hours) [39]. These data suggest a 1.7- to twofold increase in bioavailability. The area under the curve of rivaroxaban was significantly higher in subjects > 75 years versus subjects 18-45 years, while total and renal clearance were decreased [40].However, the time to maximum factor Xa inhibition and Cmax were not influenced by age.
Clinical Evaluations
Dabigatran
In a post-hoc analysis of the RE-LY trial, Eikelboom and colleagues found that patients 75 years of age and older treated with dabigatran 150 mg twice daily had a greater incidence of GI bleeding irrespective of renal function compared with those on warfarin (1.85%/year vs. 1.25%/year; P < 0.001) [29]. A higher risk in major bleeding also was seen in dabigatran patients (5.10% versus 4.37%; P = 0.07). As a result, the 2012 Beer’s Criteria lists dabigatran as a potentially inappropriate medication. An analysis was conducted of 134,414 elderly Medicare patients (defined as age > 65 years) with 37,587 person-years of follow-up who were treated with dabigatran or warfarin [44]. Approximately 60% of patients included in the analysis were over age 75 years. Dabigatran was associated with a significant reduction in ischemic stroke: HR 0.80 (CI 0.67–0.96); intracranial hemorrhage: HR 0.34 (CI 0.26–0.46); and death: HR 0.86 (CI 0.77–0.96) when compared with warfarin. As in the Eikelboom study, major gastrointestinal bleeding was significantly increased with dabigatran (HR, 1.28 [95% CI, 1.14–1.44]).
Rivaroxaban
For rivaroxaban, a subgroup analysis of patients ≥ 75 years in the ROCKET-AF trial reported similar rates of major bleeding (HR, 1.11; 95% CI, 0.92–1.34) with rivaroxaban compared with warfarin [31]. Clinically relevant non-major bleeding was significantly higher for patients aged ≥ 75 years compared with patients aged < 75 years (P = 0.01).
Apixaban
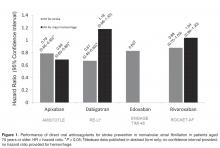
Halvorsen and colleagues found that age did not influence the benefits of apixaban in terms of efficacy and safety [47]. In the cohort of patients aged 75 years or older, major bleeding was significantly reduced compared to warfarin (HR, 0.64; 95% CI, 0.52–0.79). The safety benefits persisted even in the setting of age greater than 75 years and renal impairment. A significant reduction in major bleeding (HR, 0.35; 95% CI, 0.14–0.86) was seen in elderly patients with a CrCl; ≤ 30 mL/min (n = 221) treated with apixaban versus warfarin. Similarly, in elderly patients with a CrCl 30 to 50 mL/min (n = 1898) a significant reduction in major bleeding was reported (HR, 0.53; 95% CI, 0.37–0.76). These data are consistent with a meta-regression analysis that found a linear relationship between the relative risk of major bleeding and the magnitude of renal excretion for the DOACs (r2=0.66, P = 0.03) [48]. In this analysis, apixaban had the most favorable outcomes in terms of major bleeding compared to the other DOACs and also has the least dependence on renal function for clearance. In a pooled analysis of data from landmark trials, Ng and colleagues found that in elderly patients (defined as age > 75 years) with nonvalvular AF, only apixaban was associated with a significant reduction in both stroke and major hemorrhage (Figure 1) [49,50].
Edoxaban
Kato and colleagues performed a subgroup analysis of patients aged 75 years or older enrolled in the ENGAGE TIMI 48 study [50]. Currently the results are only published in abstract form. Regardless of treatment, the risk of major bleeding and stroke significantly increased with age (P < 0.001). An absolute risk reduction in major bleeding was reported with both 60 mg and 30 mg of edoxaban versus warfarin (4.0%/year and 2.2%/year versus 4.8%/year, respectively; no P value provided).
Therapeuti Drug Monitoring
Collectively, the data on assessment of the anticoagulant activity of DOACs using coagulation assays is evolving. These tests include but are not limited to prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin clotting time (TT), dilute TT, activated clotting time (ACT), anti factor Xa, and ecarin clotting time (ECT) assays. Although routine monitoring is not desirable, the ability to assess degree of anticoagulation in select patient populations may prove beneficial. Future studies are essential to confirm whether assessing DOAC activity using coagulation assays in vulnerable populations such as the elderly improves clinical outcomes. Several reviews on this subject matter have been published [51–55]. The reader is encouraged to review these data as there are significant limitations to currently available assays and incorrect interpretation may lead to suboptimal treatment decisions.
Renal and Hepatic Dysfunction
Depending on the specific agents, DOACs renal clearance varies from 27% to 80% [56–59]. Clinical trials often use the Cockcroft-Gault formula (CG) based on actual body weight to estimate renal function. Landmark trials evaluating the DOACs differed in their strategy for estimation of renal function using CG. For example, RE-LY and ROCKET-AF used actual body weight for the estimation of renal function, while ARISTOTLE did not specify which body weight to use. Estimation of renal function or glomerular filtration rate (GFR) by CG is frequently in discordance with actual renal function in the elderly [60]. MDRD (modification of diet in renal disease) and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) are also common estimations that provide an estimate of GFR. In a cross-sectional study, comparing the CG, MDRD, and EPI formulas in a clinical setting, data from potential kidney donors and adult patients who underwent a GFR measurement revealed that MDRD has the smallest mean bias. The influence of age was the absolute bias for estimation of renal function for all formulas. CG is additionally influenced by body weight and body mass index. When compared to CG, MDRD actually reported more accurate predictor of GFR in adults < 70 years old [61]. However, package inserts recommend dose adjustments based on estimation of CrCl using CG formula. This poses a problem in adjusting DOAC doses in elderly patients who are subject to overestimation of renal function with this antiquated equation. Among elderly patients with renal impairment, discordance between estimated and actual renal function was higher for dabigatran and rivaroxaban than for apixaban dosages [61].
Renal excretion of unchanged dabigatran is the predominant pathway for elimination (~80%) [58]. The FDA-approved dosing strategy in the US for dabigatran is 150 mg twice daily in patients with a CrCl ≥ 30 mL/min, 75 mg twice daily in patients with severe renal impairment (CrCl 15–30 mL/min), and is contraindicated in patients with a CrCl < 15 mL/min [58]. By comparison, the Canadian and the European Medicines Agency have listed patients with a CrCl < 30 mL/min (severe renal impairment) as a contraindication for use. The US-approved dosage for severe renal impairment was derived during the approval phase of dabigatran using a simulation pharmacokinetic model [62,63]. The dosage was estimated by pharmacokinetic simulation to provide similar Cmax and Cmin concentrations compared to the 150 mg twice-daily dosage in moderate renal impairment. Compared to patients with CrCl ≥ 80 mL/min, there was a 1.29- and a 1.47-fold increase in dabigatran trough plasma concentration in the CrCl 50–80 mL/min patients and the CrCl 30–50 mL/min patients, respectively. There have been many postmarketing reports of hemorrhage with dabigatran [36,84,85]. Although reporting bias is likely due to the novelty of the agent, clinicians may take key clinical pearls away from these reports. Patients often had risk factors, including low body weight, renal impairment, and polypharmacy with interacting drugs (eg, amiodarone). These risk factors are also important with the other DOACs.
A subgroup analysis of ROCKET-AF evaluating rivaroxaban 15 mg daily in patients with a CrCl of 30–49 mL/min did not identify any differences in endpoints with the exception of fatal bleeding, which occurred less often with rivaroxaban (0.28%/yr vs. 0.74%/yr; P = 0.047) [64].
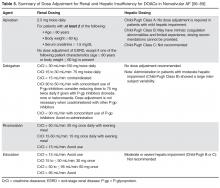
Monitoring of renal function is essential to mitigate the risk of drug accumulation. Clinicians should consider obtaining a baseline renal assessment with annual reassessments in patients with normal (CrCl ≥ 80 mL/min) or mild (CrCl 50–79 mL/min) renal impairment, and 2 to 3 times per year in patients with moderate (CrCl 30–49 mL/min) renal impairment [65]. A summary of renal dose adjustments for DOAC therapy may be found in Table 5 [56–59].
In addition to renal function, hepatic impairment can also affect the metabolism of anticoagulants. Severe hepatic impairment can lead to prolonged PT. Therefore, patients who have liver dysfunction and are treated with anticoagulation have increased risk of hemorrhagic events. Large pivotal trials on the key indications of dabigatran, apixaban, and rivaroxaban excluded patients with significant signs of hepatic impairment. Table 5 provides dosing recommendations for the different DOACs in the setting of hepatic impairment [56–59].
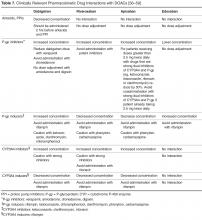
Polypharmacy And The Potential For Adverse Consequences
Costs And Cost-Effectiveness of DOACS
With the high burden of AF and the aging population, analysis of cost and value is an important consideration [76]. There are limited publications comparing the cost-effectiveness between the anticoagulation options. However, numerous cost-effectiveness studies have evaluated the individual DOACs [71–79]. Overall, the studies suggest that the DOACs are a cost-effective alternative to warfarin in the general and elderly populations. One analysis reported that dabigatran may not be cost-effective in patients with a low CHADS2 score (≤ 2) [71].
Harrington et al [80] compared the cost-effectiveness of dabigatran, rivaroxaban, and apixaban versus warfarin. This cost-effectiveness study used published clinical trial data to build a decision model, and results indicated that for patients ≥ 70 years of age with an increased risk for stroke, normal renal function, and no previous contraindications to anticoagulant therapy, apixaban 5 mg, dabigatran 150 mg, and rivaroxaban 20 mg were cost-effective substitutes for warfarin for the prevention of stroke in nonvalvular AF [80]. Apixaban was the preferred anticoagulant for their hypothetical cohort of 70-year-old patients with nonvalvular AF, as it was most likely to be the cost-effective treatment option at all willing-to-pay thresholds > $40,000 per quality-adjusted life-year gained [76,81].
Prescription costs may vary depending on payor and level of insurance. If a patient does not have prescription insurance, the annual price of generic warfarin is roughly $200 to $360, depending on dosage. Approximate annual costs for the DOACs are greater than 20 times the cost of warfarin (apixaban $4500, dabigatran $4500, and rivaroxaban $4800) [82]. However, most patients on these medications are over 65 years old and have prescription coverage through Medicare Part D. Of note, patients may have more of a burden if or when they reach the “donut hole” coverage gap. Currently, once patients spend $2960 (for 2015) and $3310 (for 2016) on covered drugs they will fall into the donut hole unless they qualify for additional assistance. At this point Medicare Part D will reimburse 45% of the cost of the newer anticoagulants since generics are currently unavailable. As a result, individual affordability may become an issue. Further complicating the scenario is the inability to apply coupon and rebate cards in the setting of government-funded prescription coverage. Clinicians should discuss these issues with their patients to help select the most valuable therapy.
Conclusions And Recommendations
Corresponding author: Luigi Brunetti, PharmD, MPH, Rutgers University, 160 Frelinghuysen Rd, Piscataway, NJ 08854, brunetti@pharmacy.rutgers.edu.