Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma





Joel Rubenstein, MD, MS
Professor, Department of Internal Medicine,
Division of Gastroenterology, University of
Michigan Medical School Director, Barrett's
Esophagus Program, Michigan Medicine,
Ann Arbor, Michigan
Disclosures: Received research grant from: Lucid Diagnostics





Barrett’s esophagus (BE) is a metaplastic transformation of the esophageal lining and the sole known precursor to esophageal adenocarcinoma (EAC), a malignancy with a 20% 5-year survival rate and about 16,000 new cases per year.1-3 Despite a lack of high-quality evidence supporting screening, guidelines suggest screening and focus heavily on endoscopy for individuals with gastroesophageal reflux disease (GERD) and other risk factors.1 Barriers to screening include reliance on GERD symptoms (given only 50% of individuals with EAC report prior GERD symptoms), provider lack of knowledge about guidelines, and the invasive nature of endoscopy.4,5 Fewer than 20% of EAC cases are detected as part of screening and surveillance.6 As many as 85% of individuals with EAC also had at least 1 missed opportunity where screening endoscopy could have been offered earlier.6
Predictive algorithms incorporating factors like age, GERD, obesity, and smoking history (e.g., Nord-Trøndelag Health Study [HUNT], Kunzmann, Kettles Esophageal and Cardia Adenocarcinoma predictioN [K-ECAN] tools) have been developed to better identify at-risk populations who should undergo screening.5,7,8 New screening modalities are also being developed. Non-endoscopic tools, such as EsoCheck with EsoGuard and Cytosponge, offer minimally invasive alternatives for detecting BE.9,10 Future efforts should focus on enhancing risk stratification, improving the referral process to screen appropriate populations, and integrating new technologies to enable earlier diagnosis and intervention, potentially improving survival outcomes for EAC.
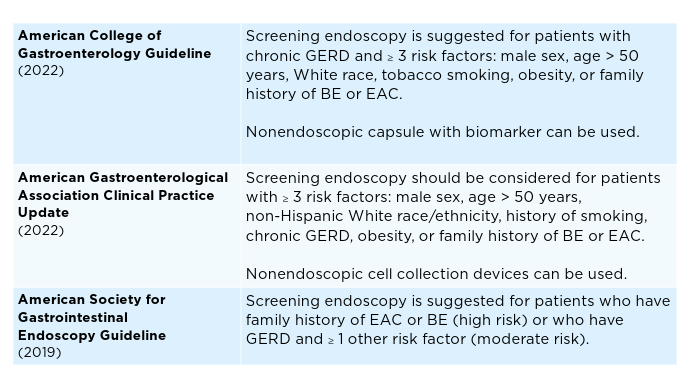 Current Screening Guidelines for BE1,11-13
Current Screening Guidelines for BE1,11-13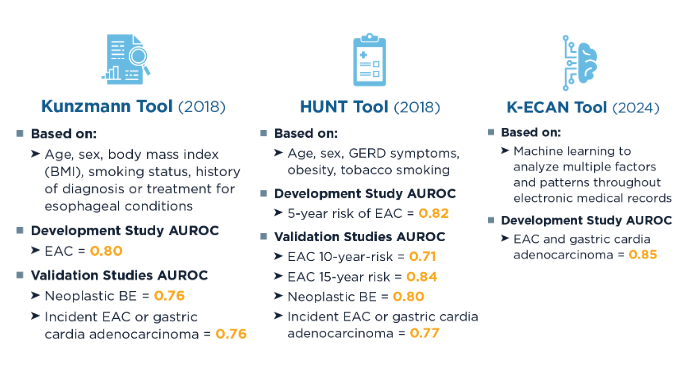 Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Screening rates for BE and EAC remain low, but predictive strategies can better identify high-risk patients for early detection strategies, including minimally invasive approaches.5,8,14 Artificial intelligence and machine learning have the potential to further refine risk prediction by integrating vast clinical datasets, but further validation is needed.8 In clinical studies, other machine learning algorithms have shown similar area under the receiver operating characteristic curve (AUROC) (0.84) to K-ECAN.8,16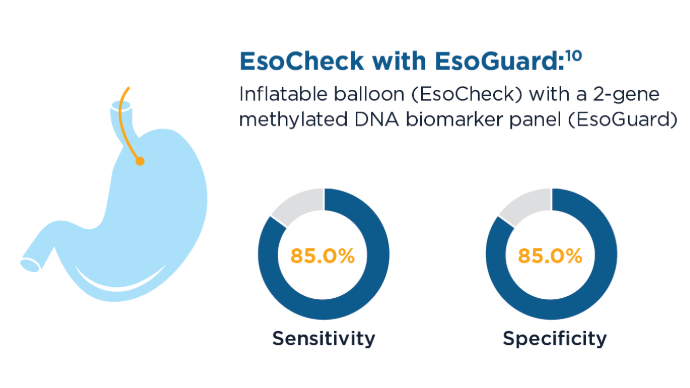 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
EsoCheck with EsoGuard and Cytosponge can be used in the primary care setting to screen patients for BE and EAC.9,10 These tools are also less invasive and faster than sedated endoscopy, with EsoCheck requiring as little as 3 minutes to collect a sample for testing.10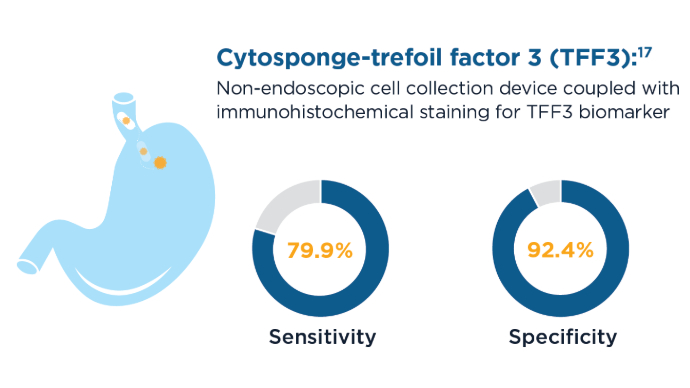 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
59% of patients who had an endoscopy after a positive Cytosponge screening were diagnosed with BE or esophago-gastric cancer. Current Screening Guidelines for BE1,11-13
Current Screening Guidelines for BE1,11-13 Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Screening rates for BE and EAC remain low, but predictive strategies can better identify high-risk patients for early detection strategies, including minimally invasive approaches.5,8,14 Artificial intelligence and machine learning have the potential to further refine risk prediction by integrating vast clinical datasets, but further validation is needed.8 In clinical studies, other machine learning algorithms have shown similar area under the receiver operating characteristic curve (AUROC) (0.84) to K-ECAN.8,16 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
EsoCheck with EsoGuard and Cytosponge can be used in the primary care setting to screen patients for BE and EAC.9,10 These tools are also less invasive and faster than sedated endoscopy, with EsoCheck requiring as little as 3 minutes to collect a sample for testing.10 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
59% of patients who had an endoscopy after a positive Cytosponge screening were diagnosed with BE or esophago-gastric cancer. Current Screening Guidelines for BE1,11-13
Current Screening Guidelines for BE1,11-13 Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Predictive Algorithms for Identifying At-Risk Patients5,7,8,14,15
Screening rates for BE and EAC remain low, but predictive strategies can better identify high-risk patients for early detection strategies, including minimally invasive approaches.5,8,14 Artificial intelligence and machine learning have the potential to further refine risk prediction by integrating vast clinical datasets, but further validation is needed.8 In clinical studies, other machine learning algorithms have shown similar area under the receiver operating characteristic curve (AUROC) (0.84) to K-ECAN.8,16 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
EsoCheck with EsoGuard and Cytosponge can be used in the primary care setting to screen patients for BE and EAC.9,10 These tools are also less invasive and faster than sedated endoscopy, with EsoCheck requiring as little as 3 minutes to collect a sample for testing.10 New Screening Modalities for BE and EAC9,10,17
New Screening Modalities for BE and EAC9,10,17
59% of patients who had an endoscopy after a positive Cytosponge screening were diagnosed with BE or esophago-gastric cancer.
Click to view more from Gastroenterology Data Trends 2025.
,false
