The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC





David Lieberman, MD, AGAF
Professor
Department of Medicine
Division of Gastroenterology
Oregon Health and Science University
Staff Physician
Department of Medicine
Portland VA Medical Center
Portland, Oregon
Disclosures:
Serve(d) as a consultant for: UDX; Geneoscopy
Received income in an amount equal to or greater than $250 from: Geneoscopy





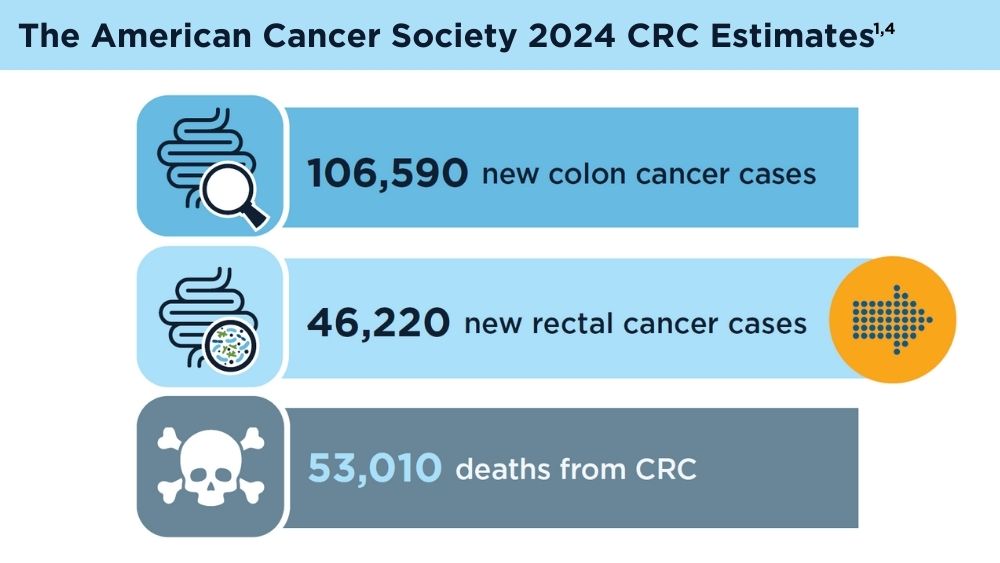
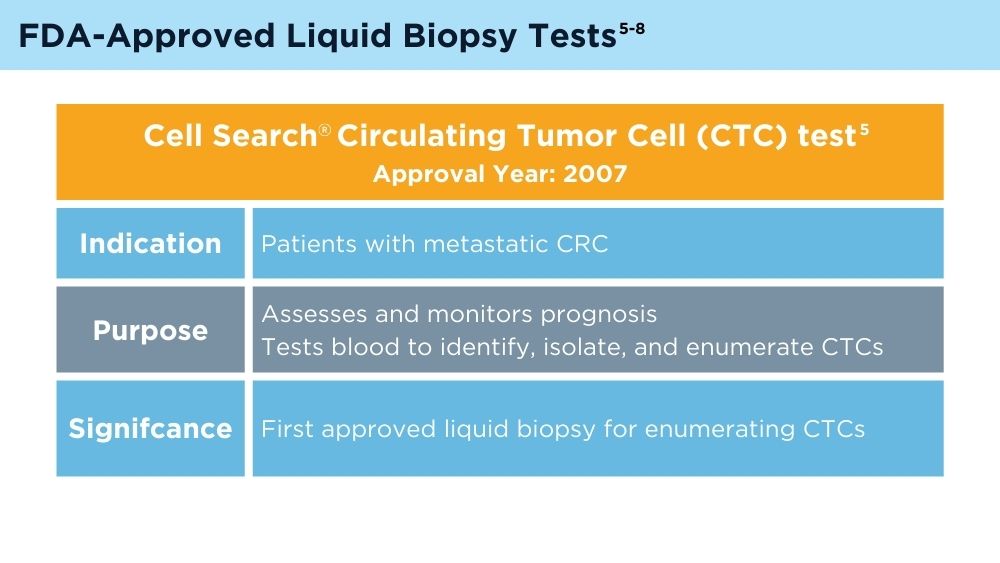
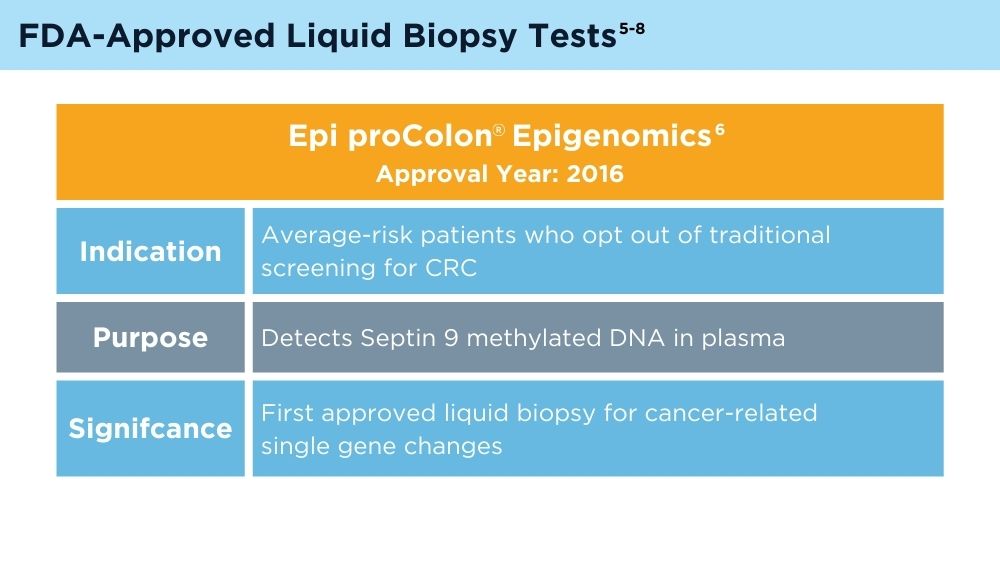
Colorectal cancer (CRC) is the third most common cancer in the United States, and early detection and monitoring are crucial for improving patient outcomes.1 Liquid biopsy (LB) is a revolutionary approach that may offer a non-invasive way to diagnose and manage CRC. The history of LB for CRC reflects a progression from early attempts to detect biomarkers in blood to the current era of precise genetic analysis using circulating tumor deoxyribonucleic acid (ctDNA) and analyzed with next-generation sequencing. The technology has significantly improved over time, leading to the potential for integration into clinical practice and to provide more personalized and effective CRC management.2
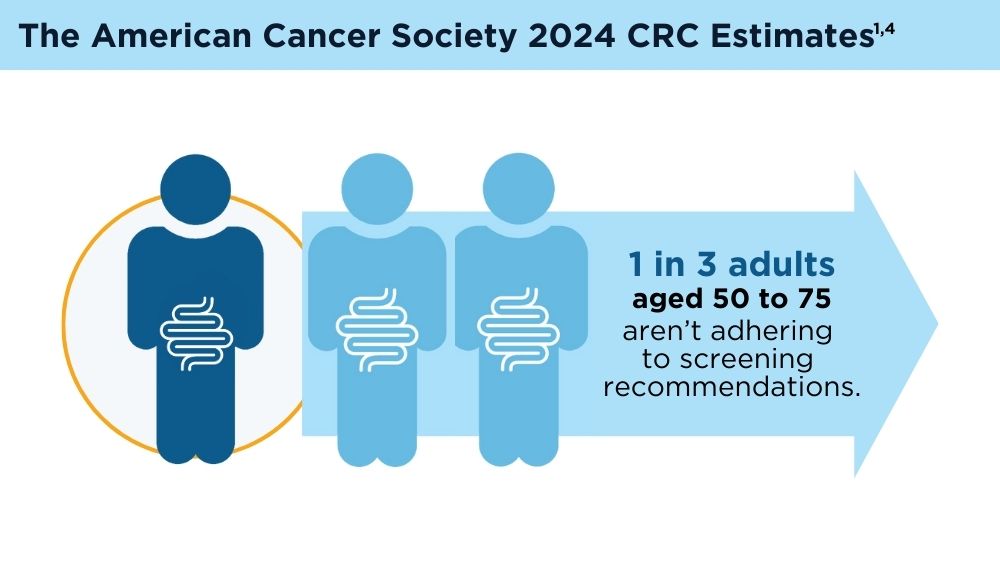
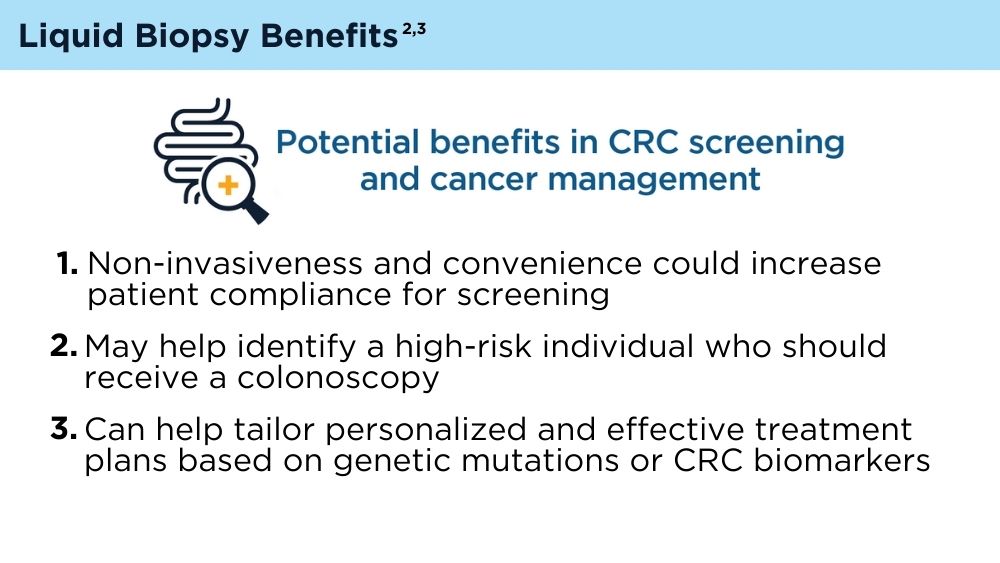
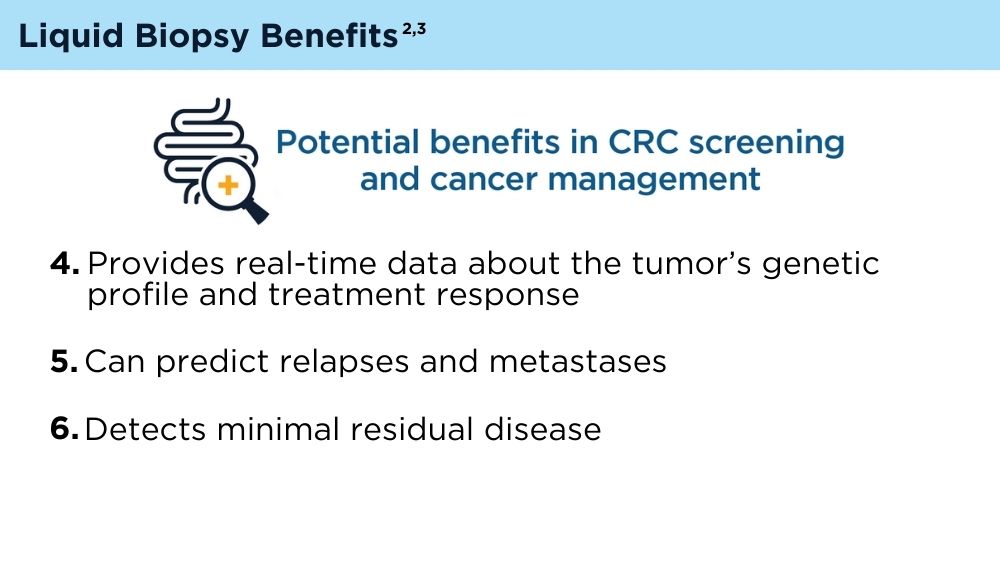
LB offers several potential advantages for CRC screening compared to traditional non-invasive screening with a stool sample, or invasive screening with colonoscopy. A blood test that could identify high-risk individuals who need colonoscopy is exciting, because it is possible that adherence to screening would be improved with LB. However, there are many challenges. Reduction of CRC mortality or incidence will depend on the ability of the test to accurately detect individuals with early-stage cancer or precancerous advanced polyps. It is not clear if the biology of such lesions would result in an adequate signal in blood if the lesion were not invasive. Test performance also depends on completion of colonoscopy if individuals have an abnormal LB. Testing methods, cost consideration, and clinical validation of performance will need to be addressed.3 As the technology advances, the role of LB in CRC screening will likely evolve and expand.