Evolution of Targeted Therapies for C difficile





Professor of Medicine
Chair GI Hospital Practice
Associate Program Director, Internal Medicine Residency Program
Medical Director Desk and Secretarial Operations
Mayo Clinic
Rochester, Minnesota





Slideshow below.
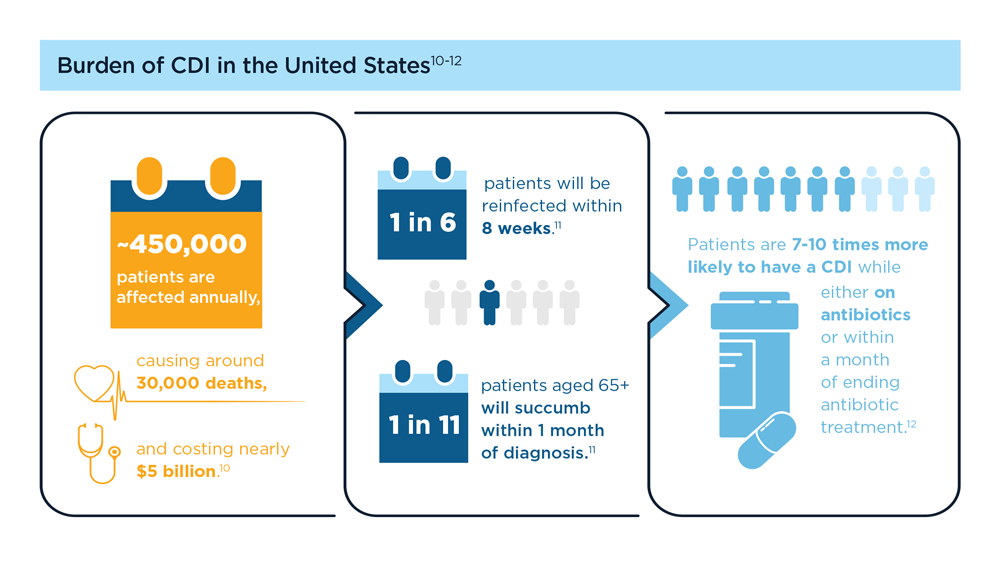
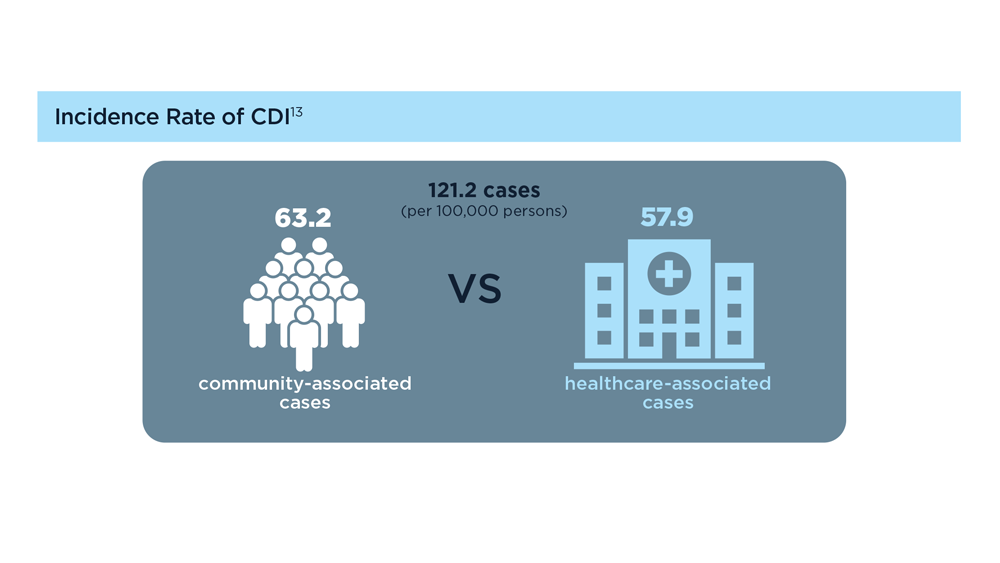
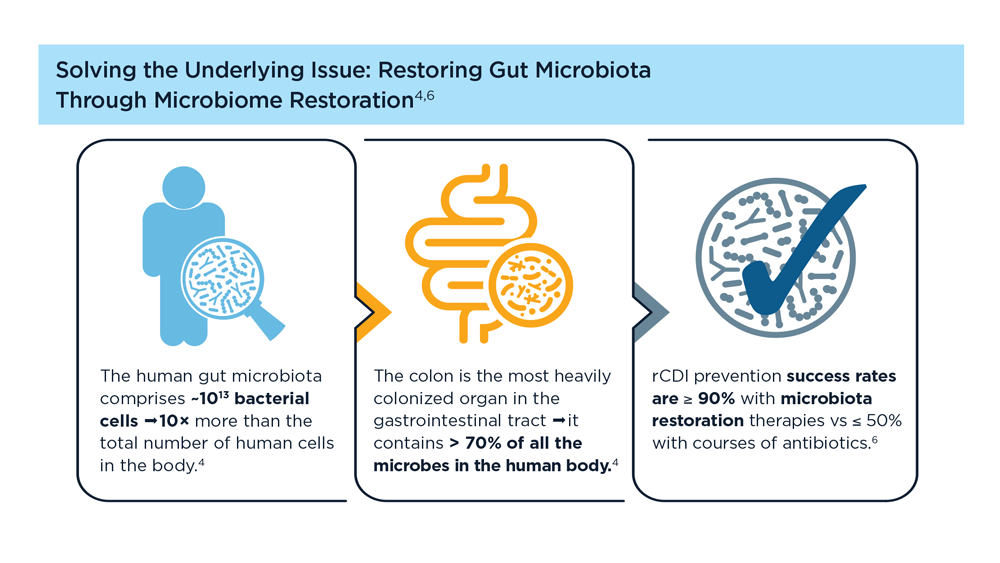
Clostridioides difficile (C difficile) is a gram-positive anaerobic bacillus that produces toxins (enterotoxin A [TcdA] and cytotoxin B [TcdB]) that can damage the lining of the gastrointestinal tract and cause potentially life-threatening disease of the large intestine.1 C difficile infection (CDI) is not only a common nosocomial infection, but an increasing incidence is seen in the community with a high disease burden, as reinfection often occurs.2 C difficile is very contagious and spreads easily via contaminated surfaces that act as reservoirs (especially in healthcare settings); the spores of this bacterium are very hard to kill.3
CDI often occurs either while the patient is taking antibiotics or soon after finishing them, as the intestinal (gut) microbiome and metabolism are altered, which allows for C difficile to proliferate.4 People with a compromised immune system or other comorbid conditions, or who are older than 65 years of age, are especially prone to CDI.1
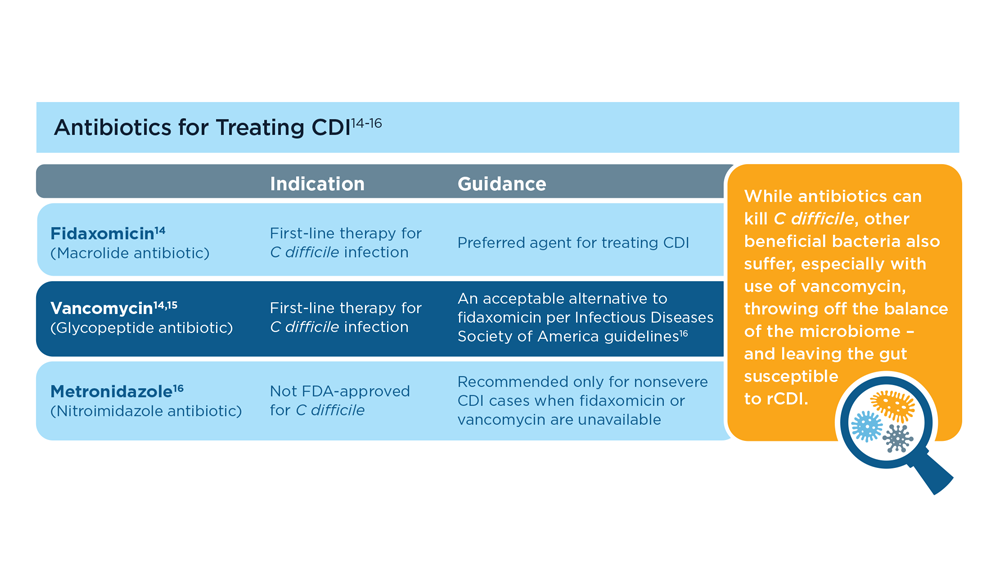
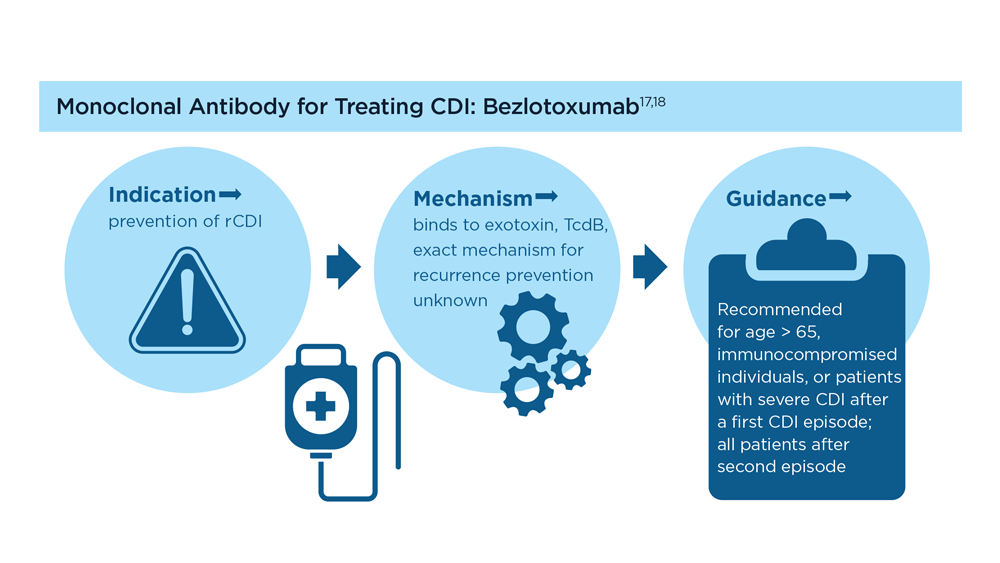
Antibiotics are the first-line treatment for primary and recurrent CDI (rCDI), although they do not always kill the spores,3 and the dysbiosis caused by antibiotics within the gut environment still needs to be addressed.4 A humanized monoclonal antibody (an immunoglobulin G against the cytotoxin B), in combination with antibiotics, has been shown to help prevent rCDI in a subset of patients.5
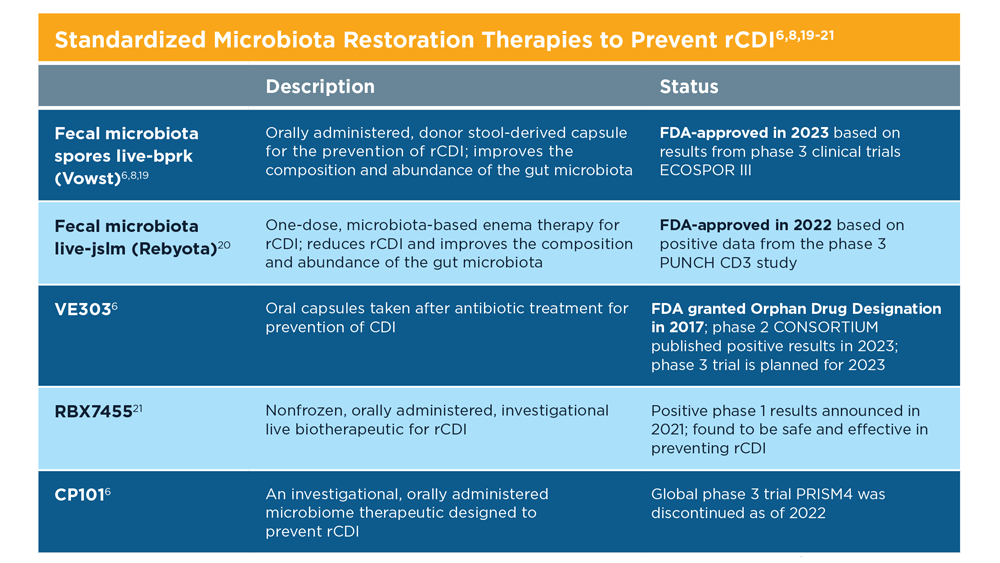
Therapies that help restore the gut microbiota to a eubiotic state, especially after antibiotic treatment for C difficile, have been shown to help manage and prevent future rCDI. Experimental fecal microbiota transplantatio (FMT) performed under enforcement discretion from the FDA is one such microbiota restoration therapy.3 Microbes harvested from healthy donor stool are transplanted into the intestine of a recipient (usually via colonoscopy) to help restore the gut microbiome and prevent CDI.7
Two therapeutics are approved by the FDA for prevention of rCDI. The first one was approved in 2022 and is a rectal administration product derived from human donor stool (fecal microbiota live-jslm [Rebyota]).8 The other is an oral capsule (fecal microbiota spores live-bprk [Vowst]) containing live spores from fecal microbiota.6 With these exciting advances, we can now begin to address additional unmet needs with future research into microbiota restoration therapies.9