Dupilumab in the Treatment of Pemphigoid Gestationis
Pemphigoid gestationis (PG) is a rare autoimmune bullous disease that is speculated to result from an excessive type 2 inflammatory immune response. It manifests as pruritic erythematous papules or urticarial plaques that progress to blistering lesions in the second or third trimester of pregnancy. Clinically and histologically, PG resembles bullous pemphigoid and is similarly associated with the presence of IgG autoantibodies against the collagenous transmembrane protein BP180. Dupilumab is a novel IgG4 human monoclonal antibody that blocks the signaling of IL-4Rα, which inhibits this Th2 inflammatory response. In this article, we highlight 2 patients with PG who were successfully treated with dupilumab.
PRACTICE POINTS
- Dupilumab inhibits the IL-4Rα subunit, which is bound by IL‐4 and IL‐13, thereby reducing type 2 inflammation associated with pemphigoid gestationis (PG).
- Dupilumab may reduce the dose and duration of systemic corticosteroid therapy for PG, and its use in the second and third trimesters of pregnancy has been supported by emerging safety data.
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.
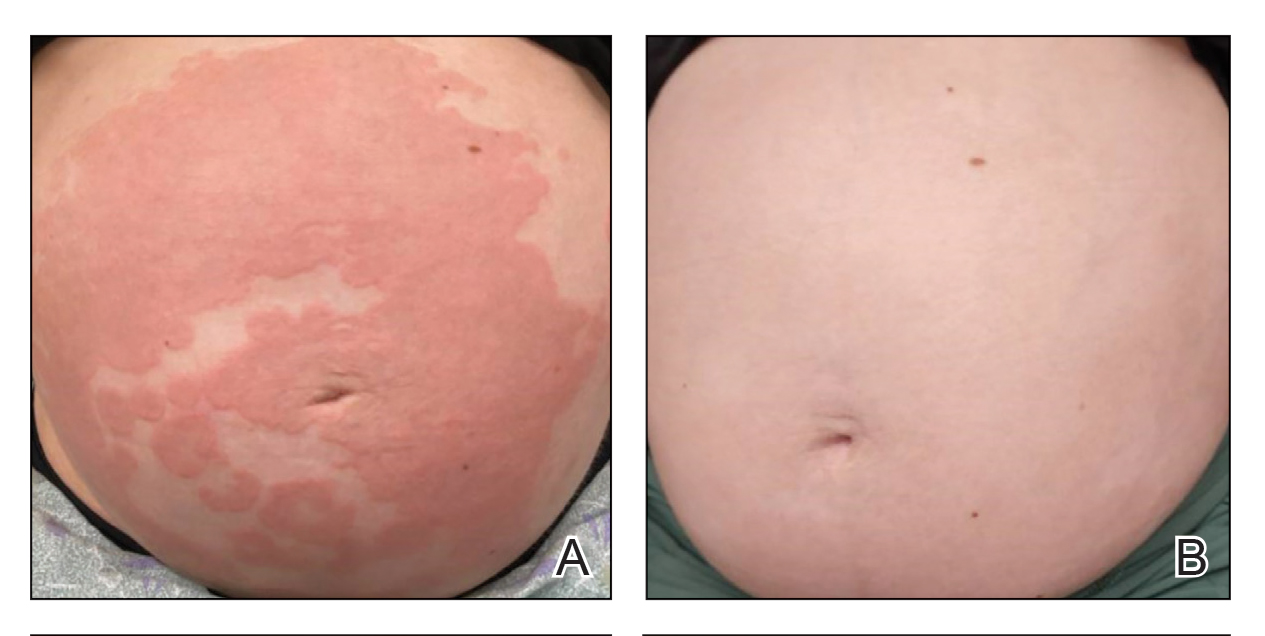
At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.
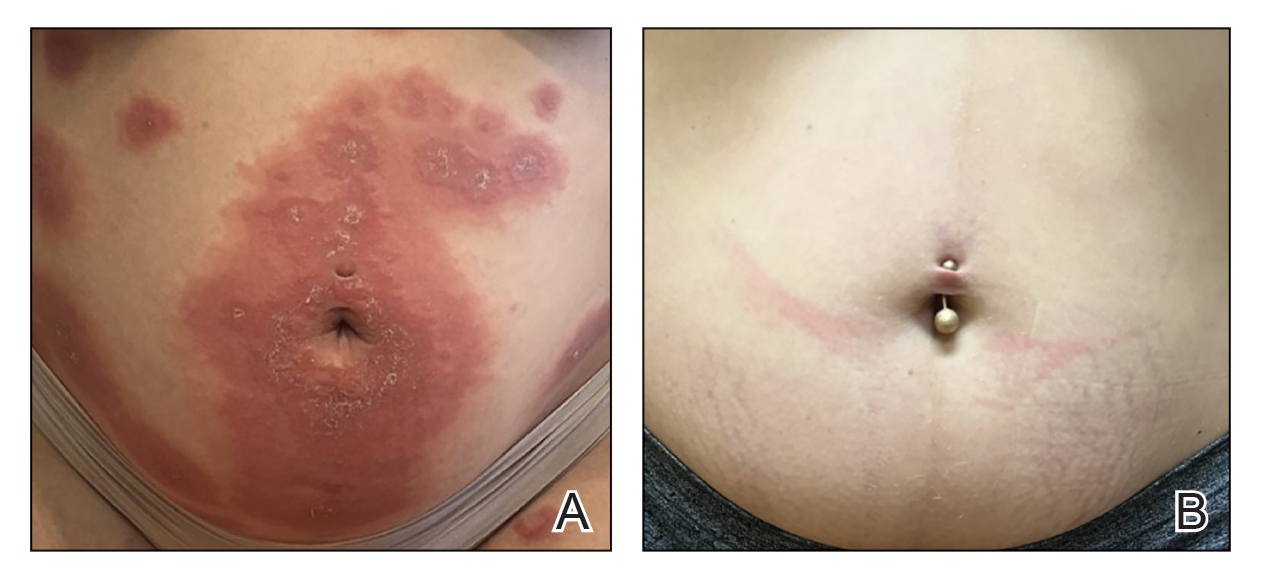
In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.




